* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lecture 30 - University of Maryland, College Park
Survey
Document related concepts
Transcript
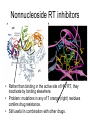
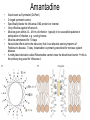
Lecture 27 Pharmacology Flint et al., Chapter 19, pp. 725 – 757. The paradox of antiviral drugs • Although we know more about the molecular biology of most viruses than about any other pathogen, we have very few drugs in our antiviral arsenal. • One of the reasons is that viruses utilize so many host functions, that to poison the virus is also to poison the host. • Need to target virus-specific mechanisms The pathway to drug discovery Fig. 19.1 1) Identify the medical need, 2) identify the mechanism, 3) devise a screen, 4) use a “screen” to identify a “lead compound” that affects the mechanism, 5) chemically alter it to increase activity, 6) then take the drug candidate through physiological and clinical characterization. Drug Discovery 1. Identify mechanism: use Mol. Biol and Genetics to identify mutants that can’t propagate virus. 2. Screen for compounds. 1. Mechanism based, e.g. inhibits HIV-1 protease 2. Cell based, e.g. inhibits virus production in cells 3. High Throughput screens. Look through hundreds of thousands of candidate compounds quickly and accurately. 4. Compound sources 1. Libraries of natural compounds 2. Computer based “Designer” compounds – structure based design 3. Genomics – use microarray technology to identify host mRNAs/proteins that are affected by virus infection…identify new antiviral gene products or toxic responses to drug candidates. 5. Combinatoral chemistry Mechanism-based screens Example: a Fluorescence based assay to monitor HIV-1 protease activity Fig. 19.14 Drug Cell-based screens Fig. 19.15 Combinatoral chemistry Fig. 19.16 •Can provide all possible combinations of a basic set of modular chemical components. •Often coupled to beads or other solid supports. •Allows active compounds to be traced, purified and quickly identified. •Thousands of compounds can be made and screened in days. Structure based drug design Squanivir (Roche) Indinavir (Merck) Protease inhibitors that were designed by modeling chemical structures on the computer to fit inside of the catalytically active site of HIV-1 protease using the HIV-1 protease X-ray crystal structure. The long road from discovery to market Fig. 19.16 •Initial screen of hundreds of thousands of compounds •Some have the desired effects •Of these, only a few aren’t totally toxic to cells •Fewer have the desired effect in animals but many of these are toxic in this context. •This pattern is repeated in humans. •Finally, after some 5 – 10 years, one compound is approved for human use. •The cost of such an enterprise typically ranges from $100M -- $500M. •Cost has significant impact on what kind of entity can do this kind of work (large corporations) and also on what diseases will be targeted for market (diseases of rich Americans). Classes of antiviral compounds • • • • • • • • Viral adsorption inhibitors Viral-cell fusion inhibitors Viral DNA polymerase inhibitors Reverse transcriptase inhibitors Protease inhibitors Neuraminidase inhibitors IMP dehydrogenase inhibitors S-adenosylhomocysteine hydrolase inhibitors Nucleoside/nucleotide analogs • Look like substrates for DNA replication • Incorporation into DNA highly mutagenic • Take advantage of the fact that viral DNA polymerases and RT’s are not as discriminating as cellular DNA pol. • Still, – – – – relatively toxic to humans, can cause mutations, teratogenic, potentially carcinogenic Examples of nucleoside analogs Acyclovir. • Specific, non-toxic • Highly effective against Herpesviruses and to some extent Varicella • Acyclic sugar group can be phosphorylated for incorporation into nucleic acids (3’ – 5’ link), but lack of 3’ OH prevents the next nucleotide from being incorporated Chain termination Acyclovir is a Prodrug: precursor to the active antiviral compound. Must first be phosphorylated by HSV thymidine kinase before it can be activated. Looks like a 5’ –OH group (i.e. acceptor) Nonnucleoside RT inhibitors • Rather than binding in the active site of HIV RT, they inactivate by binding elsewhere. • Problem: mutations in any of 7 orange (right) residues confers drug resistance. • Still useful in combination with other drugs. Protease inhibitors •The natural substrate for HIV-1 Pro is 7 amino acids long. •Ro 31-8959 mimics this structure •Fits into the HIV-1 Pro active site and doesn’t leave, inactivating the enzyme. •An example of an inhibitory suicide substrate. Amantadine • • • • • • • • Also known as Symmetrel (DuPont) 3 ringed symmetric amine Specifically blocks the influenza A M2 protein ion channel. Very effective against influenza A. Must be given within 24 – 48 hrs of infection: typically in to susceptible patients in anticipation of infection, e.g. nursing homes. Must be adminstered for 10 days Neural side effects led to the discovery that it can alleviate some symptoms of Parkinson’s disease. Today, Amantadine is primarily prescribed for nervous system disease. A methylated derivative called Rimantadine cannot cross the blood brain barrier this is the primary drug used for Influenza A. New drug targets • Inhibitors of virus entry and uncoating – e.g. Target HIV Env protein with a drug that occupies its CD4 or CCR5 binding sites. • Viral Proteases – Known inhibitors of HIV Pro. Many other viral proteases, e.g. from CMV, Hepatitis C NS 3 protein. • Virus-specific nucleic acid synthesis and processing – E.g. target RDRP’s from RNA viruses, viral proteins involved in cap snatching, synthesis of primers, recognition of RNA secondary structure, viral helicases. • Regulatory proteins – Viruses encode numerous proteins that regulate the switches between early, intermediate, and late stages of gene expression. Drug resistance • • • • Viruses have high rates of replication High rates of mutation Accelerated rates of evolution. Mutants that are more “fit” in the presence of drug will be selected for. • Drug resistant varients. – e.g. AZT resistant HIV-1 RT – Protease inhibitor resistant HIV-1 Pro – Acyclovir resistant Herpesvirus Thymidine Kinase doesn’t recognize acyclovir as a substrate.