* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Discovery and development of ACE inhibitors wikipedia , lookup
Discovery and development of cephalosporins wikipedia , lookup
NK1 receptor antagonist wikipedia , lookup
Discovery and development of non-nucleoside reverse-transcriptase inhibitors wikipedia , lookup
Discovery and development of integrase inhibitors wikipedia , lookup
Drug discovery wikipedia , lookup
Theralizumab wikipedia , lookup
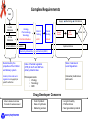
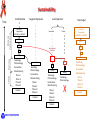
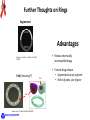
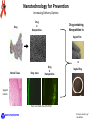
The HIV Prevention Pipeline: A Future of Possibilities IRMA Webinar August 23, 2012 Jim A. Turpin, Ph. D. Branch Chief Preclinical Microbicide and Prevention Research Branch Prevention Science Program Division of AIDS, NIAID, NIH DHHS/NIH Required Disclaimer The views expressed are those of the presenter and do not necessarily reflect the official policies of the Department of Health and Human Services (HHS), nor does mention of trade names, commercial practices, or organizations imply endorsement by the U.S. Government If your particular candidate, delivery system or prevention strategy is not presented--I apologize in advance Today I Will Address the Question: Do we currently have what it takes to create a sustainable prevention pipeline? DAIDS/PSP/PMPRB To Answer the Question I Need to Change Your Perception of What the HIV Prevention Pipeline Is Pipeline Library of Compounds >100,000 to 1,000,000 Combinations 1000 Integrase 100 CCR5 Truvada 10 UC78 CS PRO2000 BG Carraguard Savvy Preclinical TMC120 Toxicology Pharmacology Formulation Manufacturing Clinical Lead(s) TFV Phase I Phase II Phase III Phase IV Product N9 DAIDS/PSP/PMPRB Start by looking back! DAIDS/PSP/PMPRB Prevention Clinical Trials: The Good, the Bad, and the Ugly Trial Population, Regime, Drug Overall Efficacy (CI) Recalculation Comments CAPRISA 004 Women, Vaginal BAT24 1% tenofovir gel 39% (6 to 60%) Gel use: Low 28% Middle 38% High 57% HSV-1 efficacy 51% (22% -70%) iPrEx MSM Truvada , Oral, Daily 44% (15 to 63%) FEM-PrEP Women Oral, Daily, Truvada Stopped , no chance for efficacy determination No safety issues reported Partners in PrEP Serodicordant couples Oral, Daily, TDF or Truvada TDF: 62% (34 to 78%) Truvada: 73% (49 to 85%) TDF-2 Men (54%), Women (46%) Oral, Daily, Truvada 63% (21 to 83% HPTN052 Serodicordant couples Early ART CD4 350-550 Late ART CD4 >250 96% (82 to 99%) VOICE Women Daily Oral TDF or Truvada 1% Tenofovir Vaginal Gel September 16, 2011 discontinue Oral Tenofovir arm November 28, 2011 discontinue 1% Tenofovir gel arm August 13, 2012 last subject out ---reporting Q1 2013 ASPIRE Phase III 3500 Women Silicon Intravaginal Ring Dapivirine Enrollment started July 24, 2012 The Ring Study (IPM 027) 400 enrolled of 1650 DAIDS/PSP/PMPRB Verified drug use 77.9% (41 to 93%) Female: 63.6% of HIV+ endpoint Early ART superior 41% lower risk HIV Prevention in the Summer of 2012 June, 2010 July, 2011 Chinese Proverb/Curse: May You Live in Interesting Times! DAIDS/PSP/PMPRB 2012 Evolution Charles Darwin Charles Sanders Peirce (Father of Pragmatism) “It is not the strongest of the species that survives, nor the most intelligent that survives. It is the one that is the most adaptable to change.” “All the evolution we know of proceeds from the vague to the definite.” Efficacy Adherence Prevention Pipeline Basic Research Acceptability Safety The objective of the prevention pipeline must be to evolve a candidate to the safest, most effective and acceptable prevention strategy DAIDS/PSP/PMPRB Evolution of the Prevention Pipeline For pipelines the forces of evolution are also controlled by the realities of the down-selection process inherent in identifying and advancing lead candidates: Dollars versus doability--What can be accomplished with budgets and makes sense to undertake Complex preclinical, clinical and regulatory requirements Need for Sustainability—continually deliver new and improved prevention strategies DAIDS/PSP/PMPRB Dollars versus Doability: Opposing Forces 1 or 2 100 To 1,000,000 Number of Compounds I Discovery $.01 to $1.00 DAIDS/PSP/PMPRB Preclinical Virology Preclinical Studies (Critical Path) II III Clinical Studies Cost of Development Per Compound Implementation $ millions Complex Requirements R&D Supply In vitro Validation In Vivo Validation Virology Pharmacology Toxicology Preclinical Studies Clinical Testing SAFETY Clinical Testing Phase IV Studies Marketing Distribution OTC Product Consumer EFFICACY Pre formulation + formulation Implementation Chemistry, Manufacturing and Controls (CMC) Determined by the properties of the inhibitor and delivery system Code of Federal regulation (CFR) for GLP and GMP are primary determinates State, Federal and Local Regulations Federal, State and Local regulations may apply to specific activities FDA requirements • Virology • Toxicology • CMC Consumer preferences and needs Drug Developer Concerns • Meet a desired outcome • Potential for advancement DAIDS/PSP/PMPRB • • • Cost of product Ease of synthesis Marketing outlook • Long term safety • Profit and loss • Next generation products Sustainability Initial Pipeline Discovery Iterative Screen Time Targeted Expansion Library of Compounds >100,000 to 1,000,000 Lead Expansion Successful New Target Failure 1000 1000 100 100 New Limited Synthesis 10 10 Clinical Preclinical Lead(s) Toxicology Pharmacology Formulation Manufacturing Phase I Phase II Phase III Phase IV Product Lead(s) 100 Lead(s) Toxicology Pharmacology Formulation Manufacturing Phase I Phase II Phase III Phase IV Product 10 Lead(s) Toxicology Pharmacology Formulation Manufacturing Phase I Phase II Phase III Phase IV Product DAIDS/PSP/PMPRB Library of Compounds >100,000 to 1,000,000 10 Lead(s) Toxicology Pharmacology X Toxicology Pharmacology Formulation Manufacturing Phase I Phase II Phase III Phase IV Product However, we must not get lost in the complexity of the task Drugs: ARV/Non-ARV Small Molecule Peptide Protein Natural Products Nucleic Acid Other Strategies Systemic Topical Dosage Form: Pills Injectable Implantable Sustain. Release Gels/films/tablet DAIDS/PSP/PMPRB Dosing: Peri-Coital Daily Monthly Quarterly Longer? Courtesy of Joe Romano Our Question: Do we currently have what it takes to create a sustainable prevention pipeline? To answer we must examine 2 critical elements of the prevention pipeline: 1. Delivery systems 2. Candidates DAIDS/PSP/PMPRB We will look at: Delivery systems • Currently in clinical trials • Next generation- in development • The future Candidates • Now • To 2015 • 2015 to 2020 • Emerging Candidates DAIDS/PSP/PMPRB Delivery Systems Systems Currently in Clinical Trials (Phase I to III) Vaginal gels Silicon Rings Oral Tenofovir Dapivirine FACTS 001 Aspire Rectal Gels Truvada, Maraviroc, Maraviroc + FTC Maraviroc + Dapivirine HPTN 069 MTN 013/IPM 026 Tenofovir CHARM, MTN 013 DAIDS/PSP/PMPRB Injectable TMC278 LA BMGF Next of Generation Delivery Systems In Development Devices +/- Gels Other gels Vaginal Films pH transition Subliming Solid matrix Segmented Rings Rings with other polymers DAIDS/PSP/PMPRB Pod Rings Injectables Quick Dissolve Tablets Some Thoughts on Rings Where ? Polymer Options Types of Rings Matrix Silicon Ethyl Vinyl Acetate (EVA) Polyurethanes Barnhart et al. Contraception, 2005 72:196 DAIDS/PSP/PMPRB Reservoir Further Thoughts on Rings Segmented Advantages Johnson, et al. Eur. J. Pharm. Sci. 2010 39:203 Release chemically incompatible drugs Pod (Versaring™) Control drug release • Segmented-size of segment • Pod-# of pods, size of pore Pod Baum et al. J. Pharm Sci 2012 101:2833 DAIDS/PSP/PMPRB Pore Some Thoughts on Vaginal Films Water-soluble polymers designed to dissolve in the vagina and release its active ingredient Films Under Development: PMPA Dapivirine Maraviroc IQP0528 RC101 ADVANTAGES Precise and Reproducible dosage form Low unit dose cost (fractions of a penny/dose) Minimal leakage Can be used to deliver multiple active agents No applicator Scalable manufacturing process (Listerine Pocket Paks >200,000 million units sold/yr.) Courtesy of Lisa Rohan DAIDS/PSP/PMPRB Thoughts on Injectables Very Exciting Development, But------Very Limited pipeline • TMC278LA: (Janssen/BMGF) NNRTI • S-GSK1265744: (744 LA, Viiv) Integrase inhibitor Major strength of the injectible approach • Long half-life---weeks to months DAIDS/PSP/PMPRB Lots of Delivery Options, But Will Women and Men Use Them! Optimizing for delivery device acceptability/adherence Formulation Scientist Rheological parameters • Compatibility with drug • Viscosity • Osmolarity • Shearing • Stickiness • Mixing /miscibility • Color • Spreading • Coating • Adhesion to surfaces Behavioral Scientist Link Rheological with Women’s perception Identify specific formulation characteristics that yield specific women responses Handling DAIDS/PSP/PMPRB Intercourse Use Decisions • • • • • • • Perceptions Leakage Wetness Sexual pleasure Sexual comfort Removal & disposal Long residence Application Kate Morrow , Brown Univ. David Katz, UNC John Hayes, Penn. State. Univ. Greg Ziegler, Penn. State Univ. The Future! Nanotechnology DAIDS/PSP/PMPRB Nanotechnology for Prevention Increasing Delivery Options Drug Drug in Nanoparticles Drug-containing Nanoparticles in: Vaginal Film or Normal Tissue Drug alone Drug in Nanoparticles Vaginal Ring Vaginal Lumen Ham, et al. Pharm. Res. 2009 26:502 DAIDS/PSP/PMPRB Pictures courtesy of Lisa Rohan A Novel Approach for Delivery Electrospun Nanofibers A new delivery platform Multiple types of drugs Combinations Time release Biodegradable Potentially cost effective MPT compatible Courtesy of Kim Woodrow, Univ. of Washington DAIDS/PSP/PMPRB Our Question: Do we currently have what it takes to create a sustainable prevention pipeline? We have delivery systems Do we have the candidates? DAIDS/PSP/PMPRB structural formula: Candidates In Clinical Testing Next-Generation–to 2015 Single Tenofovir (TFV) NRTI, Gel Dapavirine (TMC120) NNRTI, Ring Maraviroc CCR5, Oral, Ring + Truvada Emtricitabine + Tenofovir Disoproxil Fumarate (TDF) Oral Truvada TFV Maraviroc Dapivirine gel IQP 0528 (NNRTI/Entry) TDF Ring (pod) Ring, Film, Tablet Gel, Film Film, Ring Gel, Ring Ring (pod) Combinations Maraviroc + TFV Dapivirine + TFV MIV-150 (NNRTI) + Zn IQP0528 + TFV Ring, Gel Ring, Film Gel (Carrageenan) Ring Injectable TMC278LA Nano-Liquid S-GSK1265744 (744 LA) Liquid DAIDS/PSP/PMPRB In Development 2015-2020 (Select small molecule inhibitors) Candidate Sponsor Comments RT Inhibitors 4’E-2FdA (NRTI) Michael Parniak (Univ. Pitt.) Preclinical development, highly potent memory effect Film, Ring (pod) Entry Inhibitors BMS793 (DS003) IPM Licensed from BMS gp120 Inhibitor, Preclinical development Gel, Ring Virucidal and Novel Mechanism NCp7 inhibitors PD 404,182 Ettore Appella (NCI) Targets HIV NCp7, ejecting Zn from its Zn finger, protected 5 of 6 NHP as a gel Ring A. M. Chomoun Unknown Mechanism of action -Virucidal (Texas A&M) Immune Modulatory Glycerol Monolaurate (GML) DAIDS/PSP/PMPRB Ashley Haase (Univ. Minn.) Protected in Monkeys, Phase 1 completed Gel The Protein Microbicides –An Emerging Class Candidate Sponsor Comments Protein Microbicides (Ready for clinical testing) 5P12 RANTES Mintaka Foundation (Geneva Switzerland) CCR5 inhibitor, Phase 1 in progress? Gel Griffithsin K. Palmer (Univ. Louisville) Entry inhibitor: Preclinical development, Plant produced microbicide Gel Anti-HIV mAb (4E10, VRC01) Deborah Anderson (Boston Univ. Med. School) Plant produced broadly neutralizing HIV monoclonal antibodies: Mapp Biopharmaceuticals and Reprotect collaborating Duet + Film, Ring (pod) Protein Microbicides (Formulated) RC101 (retrocyclin) Alex Cole (Univ. Florida) Entry inhibitor: Preclinical development Film, Ring (pod) PIE12 trimer Michael Kay (Univ. Utah) Highly potent HIV entry gp41 inhibitor Ring C5A Philippe Gallay (Scripps Research Inst.) Virucidal Sublimable Solid Matrix Protein Microbicides (In development) Peptide Triazoles Irwin Chaikin (Drexel Univ.) Entry inhibitor DARPins Melissa Robbiani (Population Council) Bio-optimization of naturally occurring ankyrin repeat proteins, inhibit HIV entry by binding to Env. Design alternative to antibodies DAIDS/PSP/PMPRB ON THE HORIZON OR AT THE FAR EDGE OF KNOWN SPACE Bioengineered microbicides Endogenous (vaginal, GI tract) bacteria expressing protein microbicides Immunomodulation as a prevention strategy TLR and other pattern recognition receptor inhibitors and antagonists Abasic Phosphorothiolate 2’ Deoxyribose 14-mer (PDB) Antiviral and Anti-inflammatory (Peter Katsikis, Drexel Univ.) Genetic microbicides siRNAs (multiple investigators) Adenovirus vector delivered microbicides (Wayne Marasco, Harvard Medical School) DAIDS/PSP/PMPRB Live Bacteria Delivery Systems Recent advances in Lactobacillus delivered microbicides Lagenaur et al. Mucosal Immunol. 2011 4:648 Li et al. J. Acquir. Immune Defic. Syndr. 2011 58:379 Lactobacillus bioengineered to express the HIV entry inhibitor Cyanovirin-N (CV-N) protect 4 of 12 monkeys and reduce viral load in infected Bioactive CV-N was detected in rectal secretions after feeding monkeys with bioengineered Lactobacilli in yogurt Remaining Issues 1 Immunotoxicity: Unknown in Humans if there will be immune responses to prevention protein in the bioengineered bacteria and/or loss of tolerance to endogenous bacteria 2 Colonization: Will bioengineered bacteria stably colonize without some environmental advantage, e.g. antibiotic pre-treatment 3 Regulatory Requirements: Genetically Modified Organism (GMO) 4 Human Trials: Unique trial designs will be needed to assure environment control and removal of GMO and restoration of normal microbiome in subjects DAIDS/PSP/PMPRB Our Question: Do we currently have what it takes to create a sustainable prevention pipeline? We have delivery systems We have the candidates DAIDS/PSP/PMPRB The Answer Do we currently have what it takes to create a sustainable prevention pipeline? YES—we have the necessary depth and numbers of delivery systems and prevention drug candidates to create a sustainable prevention pipeline DAIDS/PSP/PMPRB But a Sustainable Pipeline is Not a Slam Dunk Some Additional Challenges 1. What criteria(s) must the delivery system and candidate meet (down-selection) to advance to clinical testing? • Delivery formats (Gel, Film, Ring, Tablet, Injectable, Oral ) • Candidates 2. Do any of our options hold the key to global acceptability/adherence or will we need a range of delivery systems to satisfy needs and how will we manage this? 3. With devices now allowing combinations of biophysically diverse compounds, how do we manage the potential proliferation of combination strategies? 4. Roll-out and beyond challenges—How do we • Maintain supplies of drug and vehicles? • Manage the ecological and biological impact of non-biodegradable delivery devices that may contain residual drug ? DAIDS/PSP/PMPRB Final Thoughts and Take Home Messages One of our greatest prevention challenges in the next decade will not be that we lack options, but prioritizing to advance the best prevention options The prevention field is positioned to not only optimally deliver prevention strategies, but to also provide a range of delivery choices to men and women The prevention pipeline is not static and limited to only “here and now candidates”, the door is open and the infrastructure is there for continued evolution of HIV prevention strategies. DAIDS/PSP/PMPRB Acknowledgements Jim Pickett and IRMA For slides and discussions: Chelsea Polis Joe Romano Lisa Rohan Tom Smith Chuck Wira Kim Woodrow PMPRB James Cummins Anabel Lowry Leslie Marshall Cherlynn Mathias Hans Spiegel PSP Fulvia Veronese The many investigators who are making the HIV Prevention Pipeline a reality Lyric by Timbuk 3 –The future is so bright I gotta wear shades! DAIDS/PSP/PMPRB