* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Drug_Therapy_During_Pregnancy
Pharmaceutical marketing wikipedia , lookup
Compounding wikipedia , lookup
Specialty drugs in the United States wikipedia , lookup
Psychedelic therapy wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Orphan drug wikipedia , lookup
Drug design wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Drug discovery wikipedia , lookup
Neuropharmacology wikipedia , lookup
Pharmacognosy wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Prescription costs wikipedia , lookup
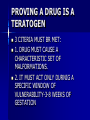
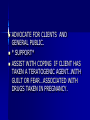
DRUG THERAPY DURING PREGNANCY Developed By D. Ann Currie , R.N.,M.S.N. PHYSIOLOGICAL CHANGES DURING PREGNANCY AND THEIR IMPACT ON DRUG THERAPY EVERY SYSTEM IN THE BODY IS EFFECTED BY PREGNANCY PHARMACOKENETICS OF DRUGS IS EFFECTED BY PREGNANCY PHARMACOKENETICS OF DRUGS DURING PREGNANCY ABSORPTION- DECREASED GI MOTILITY CAUSES INCREASED DRUG ABSORPTION. DISTURBUTION- PROTIEN BINDING IS DECREASED CAUSES INCREASED FREE DRUG TO BE AVAILABLE. METABOLISM-INCREASED HEPATIC METABOLISM OCCURS FOR SOME DRUGS PHARMACOKENETICS EXCRETION- IN THE 3RD TRIMESTER INCREASED RENAL BLOOD FLOW & GFR CAUSES SOME DRUGS TO CLEAR THE BODY FASTER. DRUG THERAPY IN THE CHILDBEARING CLIENT REQUIRES SPECIAL CONSIDERATIONS IS CHALLENGING TO PROVIDE EFFECTIVE TX WHILE AVOIDING HARM TO EMBRYO,FETUS OR NEONATE CENTERED ON RISK/BENEFIT RATIO EFFECTS OF DRUGS NOT ALWAYS KNOWN ANY DRUG TAKEN BY THE PREGNANT OR BREASTFEEDING CLIENT HAS THE POTENTIAL TO REACH THE FETUS BY WAY OF MATERNAL CIRCULATION OR NEONATE BY WAY OF BREASTMILK EFFECTS OF DRUGS ON THE EMBRYO, FETUS, OR NEONATE MAY VARY--NO EFFECT. LITTLE SERIOUS- FETAL TOXICITY SPONTANEOUS ABORTION DEATH FETAL MALFUNCTION FETAL MALFORMATIONS. DRUG THERAPY DURING PREGNACY CENTERED ON RISK/BENEFIT RATIO EFFECTS OF SOME MEDICATION ARE KNOWN UNKNOWN- NEW MEDICATIONS, DIFFERENT COMBINATIONS, DEFICIENCY IN MATERNAL METABOLISM NO DRUG IS ABSOLUTELY SAFE. RECENT STUDIES 75% OF PREGNANT CLIENTS USE 310 DIFFERENT DRUGS(PRESCRIPTION OR OTC’S) OTHER THAN VITAMINS/MINERAL SUPPLEMENTS DURING THEIR PREGNANCY. OTC’S WERE USED 4 TIMES THAT OF PRESCRIPTION DRUGS. TYPES OF DRUGS USED IN THE STUDY BY PREGNANT CLIENTS DIETARY SUPPLEMENTS ANTIEMETICS ANTACIDS TRANQUILIZERS HYPNOTICS ANTIBIOBIOTICS ANTIHISTAMINES ANALGESICS DIURETICS ETOH CNS DEPRESSANTS CNS STIMULANTS DRUG LEVELS IN THE FETUS REACHED 50100% OF THE MATERNAL BLOOD LEVELS SELF TREATMENT WITH DRUGS DURING PREGNANCY SELF TX OF MINOR ILLNESSES OR DISCOMFORTS SHOULD BE DISCOURAGED *SELFTREATMENT OF ANY ILLNESSES SHOULD BE DISCOURAGED WOMEN SHOULD BE INSTRUCTED TO KEEP A COMPLETE RECORD OF ALL MEDICATIONS TAKEN . THE CHALLENGE OF PROVIDING EFFECTIVE CARE/TX FOR THE CHILDBEARING CLIENT AVOID HARM TO EMBRYO, FETUS, NEONATE. DILEMMA UNFORTUNATELY THE RISK OF MOST DRUGS HAVE NOT BEEN ESTABLISHED. DESPITE NOT KNOWING DRUG THERAPY DURING PREGNANCY CANNOT OR SHOULD NOT BE AVOIDED BECAUSE THE HEALTH OF THE FETUS DEPENDS ON THE HEALTH OF THE MOTHER. FOR EXAMPLE: SEIZURES ,DM, MG, SLE,OR INFECTIONS. BIRTH DEFECTS INCIDENCE OF MAJOR STRUCTURAL DEFECTS(ABNORMALITIES) IS ABOUT 6% OF ALL PREGNANCIES. 3% ARE CAUSED BY DRUGS OR ENVIRONMENTAL FACTORS/EXPOSURE 3% HAVE A UNKNOWN CAUSES BIRTH DEFECTS 1/2 OF THE BIRTH DEFECTS ARE OBVIOUS AT BIRTH. 1/2 OF THE BIRTH DEFECTS AREN’T DISCOVERED UNTIL LATER IN LIFE OR DISCOVERED DURING AN AUTOPSY INCIDENCE OF MINOR STRUCTURAL ABNORMALIES IS NOT KNOWN. BIRTH DEFECTS INCIDENCE OF FUNCTIONAL ABNORMALITIES IS NOT KNOWNGROWTH RESTRICTIONS, MENTAL RETARDATION, AND LEARNING DISABLITIES SOME ABNORMALITIES HAVE MULTIPLE CAUSES-GENETIC FACTORS, ENVIRONMENTAL FACTORS, CHEMICALS OR DRUGS. TERATOGENIC TERATOGENESIS TERAS-”MONSTER” GENSIS-”PRODUCING” BIRTH DEFECTS/DISTORTION OF GROSS ANATOMY. EXAMPLES- CLEFT LIP/PALATE, CLUBFOOT, NEURAL TUBAL DEFECTS, MISSING OR MALFORMED LIMBS/FINGERS. TERATOGENIC ALSO-BEHAVORIAL AND/ OR BIOCHEMICAL ABNORMALITIES. TERATOGENESIS MAYBE DIRECT-IEMALFORMATIONS OF STRUCTURES OR INDIRECT-SUCH AS INTERFERING WITH O2 OR NUTRIENTS. TERATOGENIC EXAMPLE OF KNOWN TERATOGENIC AGENTS: ONE TIME EXPOSURE IS THALIDOMIDE-CAUSES MISSING LIMBS. CONT. OR PROLONG EXPOSURE IS ETOH-CAUSES FAS. SEE HANDOUT FOR OTHER AGENTS. FETAL EFFECTS FROM DRUGS DEPEND ON SEVERAL FACTORS TIME- WHEN DRUG IS TAKEN IN PREGNANCY. PREIMPLANTATION/PRESOMITE PERIOD-CONCEPTION TO 2 WEEK HIGH DOSE- MAYBE LETHAL/DEATH/ABORTIONS. LOW DOSE-MAYBE NOTHING. EMBRYONIC PERIOD-3-8 WEEKS *FIRST TRIMESTER* GROSS MALFORMATIONS FETAL PERIOD-9-40 WEEKS(TERM) FUNCTION PROBLEMS RATHER THAN GROSS ANATOMY*LEARNING DEFICITS &/OR BEHAVORIAL ABNORMALIES WHY IS IDENTIFICATION OF TERATOGENIC AGENTS SOMETIMES DIFFICULT TO IDENTIFY? INCIDENCE OF CONGENITAL ANOMALIES IS GENERALLY LOW. ANIMAL TESTS MAY NOT BE RELIABLE PROLONGED OR INCREASED EXPOSURE MAYBE REQUIRED. EFFECTS MAYBE DELAYED OR NOT RECONIZED. BEHAVIORAL EFFECTS ARE DIFFICULT TO DOCUMENT. CONTOLLED EXPERIMENTS CANNOT BE DONE ON HUMANS. DOCUMENTATION IS INCOMPLETE ONLY IN A LIMITED NUMBER OF DRUGS IS THE TERATOGENIC EFFECTS KNOWN OR PROVEN. LACK OF PROOF OF TERATOGENICITY DOES NOT MEAN A DRUG IS SAFE IN PREGNANCY MAY MEAN THERE IS A LACK OF RESEARCH OR INFORMATION. PROVING A DRUG IS A TERATOGEN 3 CITERIA MUST BR MET: 1. DRUG MUST CAUSE A CHARACTERISTIC SET OF MALFORMATIONS. 2. IT MUST ACT ONLY DURNIG A SPECIFIC WINDOW OF VULNERABILITY-3-8 WEEKS OF GESTATION 3.THE INCIDENCE OF MALFORMATIONS SHOULD INCREASE WITH INCREASED DOSAGE & DURATION OF EXPOSURE. PLACENTAL DRUG TRANSFER THE PLACENTA IS NOT A COMPLETE BARRIER. SOME DRUGS ARE STOPPED. SOME DRUGS(IN FACT MOST) ARE NOT. WAYS DRUGS ARE TRANSFER ACROSS- SIMPLE DIFFUSION-ACTIVE TRANSPORT. TRANSFER DEPENDS ON SEVERAL FACTORS CHEMICAL PROPERTY OF THE DRUG MOLECULAR WEIGHT. PROTEIN BINDING CAPABILITIES. CHEMICAL CONFIQURATION. LIPID SOLUBILITY.* PERIOD OF TIME DRUG REMAINS IN MATERNAL BLOODSTREAM HALFLIFE OF THE DRUG. TRANSFER DEPENDS ON SEVERAL FACTORS CONT. AMOUNT OF THE DRUG. PATHOLOGICAL PROCESSES OF THE PLACENTA. WHEN IN THE PREGNANCYINCREASED BLOOD FLOW TO THE PLACENTA IN LAST PART OF PREGNANCY. MOST DRUGS TRANSFER AND ARE AT 50-100% THAT OF THE MATERNAL LEVELS * SOME DRUG LEVELS ARE MORE THAN THE MATERNAL LEVELS DRUGS THAT TRANSFER EASILY ARE LIPID SOLUBLE. DRUGS THAT ARE DIFFICULT/HARD TO TRANSFER ARE IONIZED DRUGSHIGHLY POLAR-OR PROTEIN BOUND. HOW IS DATA COLLECTED ON DRUGS WHICH CAUSE PROBLEMS IN PREGNANCIES? NO HUMAN EXPERIMENTATION SYSTEMATIC COLLECTION AND ANALYZING OF DATA ON DRUGS TAKEN BY PREGNANT CLIENTS. REPORTING OF INFORMATION BY HEALTH PROFESSIONALS. SEE FORM. THE NURSE’S ROLE AND RESPONSIBILITY IN DRUG THERAPY IN THE CHILDBEARING CLIENT KNOWLEDGE (CURRENT &ACCURATE INFORMATION)PREGNANCY MEDICAL CONDITIONS MEDICAL TREATMENTS DRUGS AND CLIENT EDUCATION OF PREGNANT/PREPREGNAN T CLIENTS PROVIDE ACCURATE INFORMATION WITH RATIONALES INFORMATION SOULD BE CURRENT AND BASED ON EVIDENCE. * ESTABLISH ENVIRONMENT CONDUCIVE TO EXCHANCE OF INFORMATION*- TRUST. POTENTIAL HARM/RISKS. EDUCATION BENEFITS COMMON SUBSTANCES & OTC DRUGS TO AVOID IN PREGNANCYASA,ETOH,INCREASED DOSES OF MULTVITAMINS,CAFFIENE,CIGARETTE SMOKING,ETC. AVOID SELF TX-OTC’S, DRUGS FORM MEXICO. ADVOCATE FOR CLIENTS AND GENERAL PUBLIC. * SUPPORT* ASSIST WITH COPING IF CLIENT HAS TAKEN A TERATOGENIC AGENT..WITH GUILT OR FEAR…ASSOCIATED WITH DRUGS TAKEN IN PREGNANCY. PREGNANT CLIENT’S BILL OF RIGHTS “RIGHT TO KNOW” For individual drugs see handout.