* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 10, section 10.5
Survey
Document related concepts
Transcript
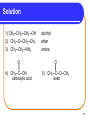
Chapter 10 Introduction to Organic Chemistry: Alkanes 10.5 Functional Groups Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 1 Elements in Organic Compounds In organic molecules, carbon atoms bond • with four bonds. • mostly with H and other C atoms. • sometimes to O, N, S, • sometimes to halogens F, Cl, and Br. 2 Functional Groups Functional groups are • a characteristic feature of organic molecules that behave in a predictable way. • composed of an atom or group of atoms. • groups that replace a hydrogen atom in the corresponding alkane. • a way to classify families of organic compounds. 3 Alkenes and Alkynes Alkenes contain a double bond between adjacent carbon atoms. Alkynes contain a triple bond. Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 4 Alcohols and Ethers An alcohol contains the hydroxyl (-OH) functional group. In an ether, an oxygen atom is bonded to two carbon atoms. –C–O–C– . R’–O–R or R–O–R R=alkyl group and R’=different alkyl group Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 5 Aldehydes and Ketones An aldehyde contains a carbonyl group (C=O), which is a carbon atom with a double bond to an oxygen atom. In a ketone, the carbon of the carbonyl group is attached to two other carbon atoms. Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 6 Carboxylic Acids and Esters Carboxylic acids contain the carboxyl group, which is a carbonyl group attached to a hydroxyl group. O ║ — C—OH An ester contains the carboxyl group between carbon atoms. Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 7 Amines and Amides In amines, the functional group is a nitrogen atom. | —N — In amides, the hydroxyl group of a carboxylic acid is replaced by a nitrogen group. Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 8 Functional Groups Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 9 Learning Check Classify each of the following as: alcohol, ether, aldehyde, ketone, carboxylic acid, ester, amine or amide. 1) CH3─CH2─CH2─OH 2) CH3─O─CH2─CH3 3) CH3─CH2─NH2 O ║ 4) CH3─C─OH O ║ 5) CH3─C─O─CH3 10 Solution 1) CH3─CH2─CH2─OH 2) CH3─O─CH2─CH3 alcohol ether 3) CH3─CH2─NH2 amine O ║ 4) CH3─C─OH carboxylic acid O ║ 5) CH3─C─O─CH3 ester 11