* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Ch 22 Organic
Survey
Document related concepts
Transcript
A group of atoms that
determines an organic
molecules’ chemical properties.
It can take the place of a
hydrogen in a hydrocarbon.
ALCOHOL
isopropanol
some properties similar to
water, able to form hydrogen
bonds.
Alcohols R-OH
• Important to industry:
– solvents, precursors to aldehydes,
ketones , org. acids & polymers
– ethanol: oldest & one of the most
important chemicals made
• fermentation: grain alcohol
• synthesized from petroleum byproducts.
Alcohols: R-OH
• Naming
– derive from alkyl name but the suffix
is ‘ol’
– use a number to tell where the
hydroxyl group is
Alcohols: R-OH
• Three Categories: determines what the
alcohol can be used to synthesize
– primary: only one carbon bonded to the
carbon with the hydroxyl group attached
– secondary: two carbons bonded to the
carbon with the hydroxyl
– tertiary: three carbons…. p 1053
Alcohols
Alcohols
Some important alcohols:
Alcohols
Alcohols
Some important alcohols:
Copyright © 2008 Pearson Prentice
Hall, Inc.
8
Alcohols: R-OH
• There are diols and triols:
– ethylene glycol = 1,2-ethandiol
• used in antifreeze
– glycerine = 1,2,3-propantriol
• used in sweets, soaps, lotions to provide
smoothness & creaminess to mixture
• used to make nitroglycerine
Alcohols
Alcohols
Some important alcohols:
ETHER
diethyl ether
volatile solvents used in
anesthetics
Ethers: R-O-R
•
•
•
•
•
Recognized by the ‘oxygen bridge’
Much less reactive than alcohols
No hydrogen bonding
Ideal for solvents
Named by the alkyl groups on either
side of the oxygen bridge
ORGANIC ACID
ethanoic acid or
acetic acid
COOH is called carboxyl group
Carboxylic Acids - R-COOH
• Uses:
– solvents, polymer synthesis, food
additives, formation of esters
• Naming:
– Use the alkyl naming rules but change the
suffix to ‘oic’ and add the word acid.
– CH3COOH = ethanoic acid p. 1057
Carboxylic Acids R-COOH
• Reactions of Carboxylic Acids
– acid-base reaction
– reduction to an alcohol
– esterification
Esters: R-COOR’
• Formed by a reaction between an
alcohol and a carboxylic acid
• Naming:
– use alcohol prefix and org. acid root
with suffix changed to ‘ate’.
– “alkyl carboxylate”
Esters RCOOR’
• Properties/Reactions
– pleasant odors, used as artificial
flavorings in foods, medicines, etc.
– not very reactive except with strong
bases.
• Saponification: triglyceride/fatty acid
with NaOH to make soap.
Saponification
• Soaps are mixtures of sodium or
potassium salts of fatty acids which can
be derived from oils or fats by reacting
them with an alkali (such as sodium or
potassium hydroxide) at 80°–100 °C in a
process known as saponification.
• http://www.edinformatics.com/interactive_molecules/soap.htm
Saponification
CH2
CH
O
O C R
O C R'
O
CH2 O C R''
O
a fat or oil
R C O- K+
O
CH2 OH
KOH
H2O
CH
OH
+
R'
C O- K+
O
R''
C O- K+
O
soap
CH2 OH
glycerol
Saponification
• fat + NaOH ---> glycerol + sodium salt of fatty acid :
– CH2-OOC-R - CH-OOC-R - CH2-OOC-R (fat) + 3 NaOH (
or KOH)
• both heated --->
– CH2-OH -CH-OH - CH2-OH (glycerol) + 3 R-CO2-Na (soap)
R=(CH2)14CH3
How Soap Works
Soap is a surface active agent referred to as a
surfactant. All surfactants are molecules that contain
both polar regions and non-polar regions. The polar
regions are hydrophilic ("water loving"), while the nonpolar regions are hydrophobic ("water fearing") or
lipophilic ("fat loving"). The polar regions will readily
dissolve in water, while the non-polar regions will tend
to associate with each other. Oils and fats, which do
not dissolve in water, will also associate with the nonpolar regions of the surfactants. This process results in
dispersion of these molecules leading to the cleaning
properties that we associate with soap.
http://www.edinformatics.co
m/interactive_molecules/so
ap.htm
Chapter 12 Section 2 The Solution
Process p. 407-417
21
Insert Holt Disc 2
Saponification
• Sodium stearate dissolves in water but
calcium stearate does not = soap scum.
• Hard water contains Ca2+, Fe3+, Mn2+, etc.
• How to “soften” water:
distillation, ion exchange (water softener
systems), precipitation (Calgon)
ALDEHYDE
methanal or
formaldehyde
C=O group is called carbonyl
group
Aldehydes R-C=O
• Carbonyl group is always at the
end of the carbon chain.
• Naming: use alkyl prefix and root,
change suffix to ‘al’.
• Uses: flavorings -vanillin,
cinnamaldehyde, polymer
synthesis.
Aldehydes R-C=O
• Can be extracted from natural sources:
vanillin from the vanilla bean
• Can be synthesized from the oxidation
of a primary alcohol
– see p. 1056
KETONE
propanone or
acetone
polar solvents used in paints
and textile processing
Ketones R-C=OR’
• Carbonyl is found between at least two
carbons in the chain.
• Named as alkyl with suffix of ‘one’
• Simplest ketone is propanone
• Produced commercially by the
oxidation of a secondary alcohol p1056
Aldehydes and Ketones
AMINE
methylamine
slightly basic compounds with
some similarities to ammonia,
often have an unpleasant, fishy
smell
Alcohols, Ethers, and Amines
Amines
Amines – R-NH2
• Naming Amines
– Simple amines: name the alkyl group
attached to the nitrogen and add the
word amine: CH3NH2 = methyl amine
– Complex amines: The NH2 group is
treated like a branch and is called an
amino group: CH3CH2CH2CH2NH2 =
1-aminobutane
Large molecule
made of many
repeated small
subunits, each of
which is a small
molecule or
group of atoms.
2 carbons
H
double bond
H
many
POLYETHYLENE
C
C
H
H
ethylene = monomer
(C2H6)n
Synthetic Polymers
• Small units are called monomers. A
polymer can be made from the same or
different monomers.
• A polymer made from two or more different
monomers is called a copolymer.
• Natural polymers: proteins, carbohydrates,
rubber
• Synthetic polymers: plastics, fibers and
elastomers.
Synthetic Polymers: addition
Polymers: Large molecules formed by the repetitive
bonding of many smaller molecules, called monomers.
Addition Polymerization
01
• Simplest polymer is polyethylene, made from
“stringing together” ethylene (ethene)
molecules.
• The molecule consists of over 500 units. These
repeating units are represented below where
one of the two bonds in the double bond in
CH2=CH2 has been broken and the two paired
H H
electrons split to form two new bonds.
*
C
C
H
H
*
n
Addition Polymerization
07
H
H
C
H3C
CH
C
H
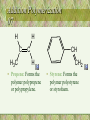
• Propene: Forms the
polymer polypropene
or polypropylene.
CH2
• Styrene: Forms the
polymer polystyrene
or styrofoam.
Addition Polymerization
08
F
F
C
F
H
C
H
C
F
H
C
Cl
• Tetrafluoroethene:
• Chloroethene:
Forms the polymer
Commonly known as
polytetrafluoro-ethene.
vinyl chloride this
This polymer is
forms the polymer
marketed as PTFE,
polyvinylchloride or
Teflon, and Goretex.
PVC.
Addition Polymerization
09
H
H
H
CH3
C
C
H
C
C
H
CN
• Cyanoethene:
Commonly known as
acrylonitrile this forms
the polymer
polyacrylonitrile.
C
O
O
CH3
• Methyl 2-methyl-2propenoate:
Commonly methyl
methacrylate this
forms the polymer
PMMA.
Condensation Polymerization
01
• In condensation reactions, two molecules
link by forging bonds between their
functional groups.
• In the process a molecule of H2O is formed.
• Polymerization may take place between two
different functional groups, or two that are
the same.
Synthetic Polymers: condensation
Polymerization:
Condensation Polymerization
03
H
N
• Kevlar:
• A Polyamide
where Hydrogen
O
H
N
N
O
N
H
H
N
O
O
O
H
N
O
O
O
H
N
O
O
N
H
N
H
O
N
H
N
H
H
N
O
O
N
H
H
N
bonds increase
strength.
H
N
H
O
N
O
Condensation Polymerization
04
• Polyesters: Account for over 40% of the
more than 4 billion kg of synthetic fibers in
the USA.
O
O
HO
O
OH +HO
O
OH
O
Terephthalic Acid
+ H2O
O
Ethylene Glycol
PET
n
Synthetic Polymers
Properties of Polymers 01
• Plastics: A polymeric material that hardens
on cooling or on evaporation of solvent,
allowing it to be molded or extruded into
specific shapes or spread into thin films.
• Plastics fall into two groups:
1. Thermoplastic – melt or deform on heating.
2. Thermosetting – retain structure on heating.
Properties of Polymers 02
• Thermoplastic polymers: have very little
or no cross-linking between the polymer
chains. Cross-linking provides the structure
and thermal stability.
Properties of Polymers 03
• Thermosetting Polymers: Have extensive
cross-linking that is derived from either the
component molecules or from some
secondary process (vulcanization).
Properties of Polymers 05
• Fibers: Thin threads of polymers made by
forcing a fluid thermoplastic material through
tiny pores.
• Most synthetic fibers are polyesters, polyamides,
or polyacrylonitrile.
• Polar functional groups in these polymers
produce strong intermolecular forces that add
significant tensile strength to the material.
Properties of Polymers 06
• Elastomers: A polymer that is flexible,
allowing it to be distorted from one shape to
another.
• Polyisoprene (natural rubber), polybutadiene,
and butadiene-styrene copolymers are
important.
• All contain some C=C bonds.
cis -Isoprene
unit
trans - Isoprene
unit
Polymers
• What are the monomer(s) used to produce
each addition polymer below?
• 1. -CH2CH(CN)CH2CH(CN)• 2. -CH2CF2CH2CF2• 3. -CH(OH)CH(OH)CH(OH)CH(OH)• 4. -CH2C(CN)(CH3)CH2C(CN)(CH3)-
Polymers
• What are the repeating units in the
condensation polymers formed from the
monomer(s) below?
• 1. HO2C(CH2)2CO2H and HOCH2OH
• 2. HOCH2CH2CO2H
• 3. H2N(CH2)2CO2H
• 4. H2NCH2NH2 and HO2CCH2CO2H