* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Carbon Chemistry
Survey
Document related concepts
Transcript
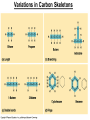
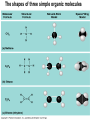
Chapter 4 Carbon and the Molecular Diversity of Life 1 • Carbon is the backbone of biological molecules (macromolecules) • All living organisms are made up of chemicals based mostly on carbon 2 • Organic chemistry is the study of carbon compounds • Carbon atoms can form diverse molecules by bonding to four other atoms • Carbon compounds range from simple molecules to complex ones • Carbon has four valence electrons and may form single, double, triple, or quadruple bonds 3 • The electron configuration of carbon gives it covalent compatibility with many different elements Nitrogen Carbon Hydrogen Oxygen (valence = 1) (valence = 2) (valence = 3) (valence = 4) H O N C Figure 4.4 4 Variations in Carbon Skeletons 5 The shapes of three simple organic molecules 6 • The bonding versatility of carbon allows it to form many diverse molecules, including carbon skeletons Name and Comments (a) Methane Molecular Structural Formula Formula Ball-andStick Model SpaceFilling Model H CH4 H C H H (b) Ethane C2H 6 (c) Ethene Figure 4.3 A-C (ethylene) C2H4 H H H C C H H H H H C C H H 7 • Hydrocarbons are molecules consisting of only carbon and hydrogen • Hydrocarbons are found in many of a cell’s organic molecules Fat droplets (stained red) Figure 4.6 A, B (a) A fat molecule 100 µm (b) Mammalian adipose cells 8 • Isomers are molecules with the same molecular formula but different structures and properties (ex: glucose and fructose) • Three types of isomers are –Structural –Geometric –Enantiomers 9 Three Types of Isomers 10 • Enantiomers are important in the pharmaceutical industry igure 4.8 L-Dopa (effective against Parkinson’s disease) D-Dopa (biologic ally inactive) 11 • Six functional groups are important in the chemistry of life –Hydroxyl –Carbonyl –Carboxyl –Amino –Phosphate –Sulfhydryl –ALL OF THE ABOVE ARE POLAR AND HYDROPHILIC! –Methyl (nonpolar and hydrophobic) 12 Functional Groups of Organic Compounds pp. 64-65 13 • Functional groups are the parts of molecules involved in chemical reactions • They give organic molecules distinctive chemical properties Figure 4.9 Estradiol OH CH3 HO Female lion OH CH3 CH3 O Testosterone Male lion 14 Some important functional groups of organic compounds FUNCTIONAL GROUP HYDROXYL CARBONYL O OH (may be written HO STRUCTURE Figure 4.10 CARBOXYL C C OH ) In a hydroxyl group (— OH), a hydrogen atom is bonded to an oxygen atom, which in turn is bonded to the carbon skeleton of the organic molecule. (Do not confuse this functional group with the hydroxide ion, OH–.) O The carbonyl group ( CO) consists of a carbon atom joined to an oxygen atom by a double bond. When an oxygen atom is double-bonded to a carbon atom that is also bonded to a hydroxyl group, the entire assembly of atoms is called a carboxyl group (—COOH). 15 Some important functional groups of organic compounds NAME OF COMPOUNDS Alcohols (their specific names usually end in -ol) EXAMPLE H H H C C H H Ketones if the carbonyl group is within a carbon skeleton Aldehydes if the carbonyl group is at the end of the carbon skeleton H OH Ethanol, the alcohol present in alcoholic beverages H H C H C H H Acetone, the simplest ketone H Figure 4.10 H O C H Carboxylic acids, or organic acids H H C C H H C H O C OH Acetic acid, which gives vinegar its sour tatste O C H Propanal, an aldehyde 16 Some important functional groups of organic compounds AMINO SULFHYDRYL H N H The amino group (—NH2) consists of a nitrogen atom bonded to two hydrogen atoms and to the carbon skeleton. Figure 4.10 PHOSPHATE O SH (may be written HS ) O P OH OH The sulfhydryl group consists of a sulfur atom bonded to an atom of hydrogen; resembles a hydroxyl group in shape. In a phosphate group, a phosphorus atom is bonded to four oxygen atoms; one oxygen is bonded to the carbon skeleton; two oxygens carry negative charges; abbreviated P . The phosphate group (—OPO32–) is an ionized form of a phosphoric acid group (—OPO3H2; note the two hydrogens). 17