* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Biehl_Chapter 20
Survey
Document related concepts
Transcript
EXAMPLE
H N-CH
3
O
+
+
+
N
HOH
CH3
CH3
N
N
Na(MeCOO)3BH
H
CH3
Other Ways to Prepare Primary
amines
REDUCTION OF NITROANILINES - as before
REDUCTION OF NITRILES
-1
CN
RCH2X
RCH2CN LAH RCH2CH2NH
SN2 conditions
essentially replaces halogen with a CH2NH2
group.
Increases carbon chain length by one carbon
Don’t do problem 27b ch.19
Please do problem 30b,
REDUCTION OF AZIDES
-1
N
3
RCH2X
RCH2N3
LAH
RCH2NH2
SN2 conditions
essentially replaces halogen with an NH2 group.
No increase in carbon chain length
NOTE 1-bromopentane to 1-pentanamine - azide
but
1-bromopentane to1-hexanamide - CN-
Chapter 20 Carboxylic Acids
RCOOH COOH IS A CARBOXYL GROUP
RCOOH
- H+
RCOO
CARBOXYLATE
NOMENCLATURE
COMMON
HCOOH
MeCOOH
FORMIC
Acetic
EtCOOH
Propionic
IUPAC
METHANOIC ACID
Prop-COOH BUTYRIC
4
3
2
g
b
a
ETHANOIC ACID
PROPANOIC ACID
BUTANOIC ACID
1
NH2 CH2CH2CH2COOH
gAminobutyric Acid
4-Aminobutanoic
Acid
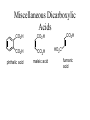
Nomenclature of Dibasic Acids
HOOC-COOH
HOOCCH2COOH
Oxalic acid
Malonic acid
HOOC(CH2)2COOH
Succinic acid
HOOC(CH2)3COOH
Glutaric acid
HOOC(CH2)4COOH
Adipic acid
HOOC(CH2)5COOH
Pimelic acid
Miscellaneous Dicarboxylic
Acids
CO2H
CO2H
CO2H
CO2H
phthalic acid
maleic acid
CO2H
HO2C
fumaric
acid
Acidity of Carboxylic Acids
pKa 4-5
STRONGER THAN ALCOHOLS -RESONANCE
SUBSTITUENT EFFECTS
ALIPHATIC ACIDS - SAME AS ALCOHOLS
O
O
O
F
OH
Cl
pKa = 2.86
F
OH
OH
F
pKa = 2.59
F
pKa = 0.23
Substituent Effects in Aromatic
Acids
CO2-
CO2-
CO2-
OMe
NO2
pKa = 4.19
pKa = 3.41
pKa = 4.46
In general, o.p directing groups decrease acidity and meta directing groups
increase acidity.
CO2-
CO2-
CO2-
CO2-
O
O
+
N
O + O
O
N
+
O
stabilizes carboxylate ion
Me
Me +
destabilizes carboxylate ion
Salts of Carboxylates
FORMATION OF CARBOXYLATES
RCOOH
+ NaHCO3
RCOO-
+
H2CO3
-HOH
CO2
RECALL - C6H5OH DOES NOT REACT WITH NaHCO3
Carboxylates by Saponification
of lipids
O
CH2O C R
CHO C
O
O
O C R
OHR'
Heat
CH2OH
+
O CH R'
O
+
CHOH
+
CH2O C R"
O
LIPID
O C R"
O
SOAP
Common R groups are C3, C15, C17 and
can be saturated or unsaturated
CH2OH
GLYCEROL
HYDROGENATION OF OILS
OIL + H2
CAT
FAT
MARGARINE
In clinical studies, trans fatty acids or hydrogenated
fats tend to raise total blood cholesterol levels
and LDL ("bad") cholesterol and lower HDL
(“GOOD”) cholesterol when used instead of cis
fatty acids or natural oils. These changes may
increase the risk of heart disease.
Spectroscopy
IR - Broad OH 3300-2800 cmMS McLafferty if delta hydrogen present
1-H NMR delta around 12-13ppm
13-H NMR delta around 200 ppm
PREPARATION OF ACIDS
CARBONYLATION OF ORGANOMETALLICS
O
R--Li +
PhLi +
C
O
O
C
O
O
RC
O
Ph C
+
H
O-
H+
O-
O
RC
OH
O
Ph C
OH
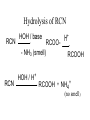
Hydrolysis of RCN
RCN
HOH / base
+
H
RCOO-
- NH3 (smell)
RCOOH
+
RCN
HOH / H
RCOOH
+
NH4+
(no smell)
Basicity of amines
The greater the availability of the lone pair electrons on nitrogen, the greater the base.
In the old days, pKb was a measure of base strength.
Kb = [RNH3+] [OH-] / RNH2
pKb = - log Kb
The stronger the base the lower the pKb
EFFECTS ON AMINE BASICITY
1. INDUCTIVE EFFECT - ALKYL SUBSTITUTION
CH3
CH3-NH2 < CH3-NH
3.36
3.28
METHYL GROUP INCREASES ELECTRON DENSITY ON N
2 METHYLS ARE BETTER THAN ONE
WATCH OUT THREE METHYL GROUPS DECREASES
BASICITY pKb = 4.26 - Steric inhibition of solvation of HOH
with the NH+ of the R3NH+ cation.
2. RESONANCE EFFECT
Base weakening Why?
+
NH2
Delocalizes electron pair on N!!
NH2
NH
+
vs.
-
N
3.3
9.4
aromatic 6 pi system
H
H
N
H
not aromatic
pKb = 15
Hybridization
The greater the % of s character The closer the lone pair is to N The weaker the base
N
sp2
8.75
H
N
H3C
sp3
2.88
N:
sp
24
REACTIONS OF AMINES
Alkylation of amines by alkyl halides -
Only two situations of importance
Excess ammonia - stops are monoalkylation stage
PhCH2Br
+
excess NH3
PhCH2NH2
Excess Methyl iodide - all the way to quaternary salt
Propyl-NH2
+ ex MeI
Propyl-N(Me)3+
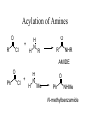
Acylation of Amines
O
R
Cl
+
H
H
N
O
R
R
NHR
AMIDE
O
Ph
Cl
+
H
H
N
O
Me
Ph
NHMe
N-methylbenzamide
Mechanism
O
Ph
+
Cl
H
H
N
O-
H+
Me
Ph
Cl
NHMe
O
Ph
NHMe
Amines as Leaving Groups
Hofmann Elimination
R-NH2
H
excess MeI
+
R-NMe3 I-
OH-
Ag2O
+
R-NMe3 OH-
HEAT
-N(Me)3
-HOH
N(Me)3
ANTI ELIMINATION - Less stable (less substituted) alkene
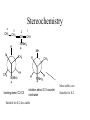
Stereochemistry
4
CH3
3
2
CH2
CH
H
H
CH3
1
CH3
N(Me)3
+
Me
CH3
H
CH3
H
N(Me)3
H
+
+
looking down C2-C3
N(Me)3
H
rotation about C-3 counter
clockwise
Suitable for E-2;less stable
More stable; not
Suitable for E-2
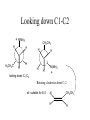
Looking down C1-C2
+ N(Me)3
H
H3CH2C
CH2CH3
H
H
H
H
H
H
H
looking down C1-C2
N(Me)3
+
Rotating clockwise about C-2
all suitable for E-2
H
CH2CH3
H
H
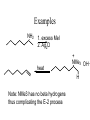
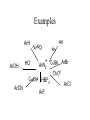
Examples
NH2
1. excess MeI
2. Ag2O
heat
+
NMe3 OH_
H
Note: NMe3 has no beta hydrogens
thus complicating the E-2 process
Another example
H
N
Me2
N+
1. excess MeI
2. Ag2O
HO-
H
heat
NMe2
Me2N
H
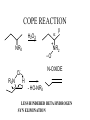
COPE REACTION
a
H2O2
NR2
_
+
NR2
O
N-OXIDE
-
O
R2N
b
H
- HO-NR2
LESS HINDERED BETA HYDROGEN
SYN ELIMINATION
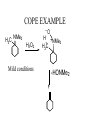
COPE EXAMPLE
NMe
2
H3C
H2O2
Mild conditions
-O
H +NMe
2
H2C
-HONMe2
Reaction of Primary Amines with
HNO2
Preparation of HNO2
NaNO2
+
HCl
HNO2 + NaCl
Reaction
Aliphatic amines
RNH2
NaNO2
HCl
RN2+
R+
-N2
SN1
RN2+
diazonium ion
R+
Hot carbocation
mixture of products
Aromatic Primary Amines
ArNH2
NaNO2
HCl
0-5
oC
ArN2+
stable
undergoes SN1 with
nucleophiles
Examples
ArI
ArH
K
I
3
O
P2
H
ArOH
HO-
+ CuBr ArBr
ArN
2
CuCl
CuCN HBF4
ArCN
ArF
ArCl
Conversion of NO2 to NH2
Good way to introduce NH2
ArH
HNO3
H2SO4
ArNO2
RED
ArNH2
RED = SnCl2/ HCl
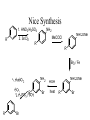
Nice Synthesis
1. HNO3/H2SO4
2. SnCl2
R
NH2
NHCOMe
MeCOCl
R
R
Br2 / Fe
1. NaNO 2
HCl
R
2. H 2 PO 3 / ROH
R
Br
NH2
HOH
Br
heat
NHCOMe
R
Br
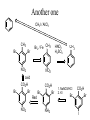
Another one
CH3I / AlCl3
CH3
Br
Br
Br
Br2 / Fe CH3
NO2
NO2
oxid
CO2H
Br
CO2H
Br
Br
Red
NO2
NH2
HNO3
H2SO4
CH3
1. NaNO3/HCl
2. KI
Br
CO2H
Br
I
Synthesis of Amines
Reductive Amination
R
1. C
N H
O
R'
2. Reduction
R
N CH
R'
NOTE: carbonyl has been reduced
and aminated - most general method
Examples
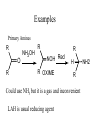
Primary Amines
R
R
O
R
NH2OH
R
NOH Red
R OXIME
H
R
Could use NH3 but it is a gas and inconvenient
LAH is usual reducing agent
NH2
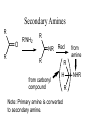
Secondary Amines
R
O
R
R'NH2
R
NR
Red
R
from carbonyl
compound
from
amine
R
H
Note: Primary amine is converted
to secondary amine.
R
NHR
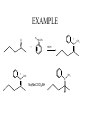
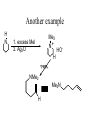
Tertiary Amines
R
R'R"NH2 R
O
R
+
NR'R"
R
iminium salt
Very unstabile
So reaction is run with reducing presence at all times.
This means that there will both product
and carbonyl compounds presence.
Must use reducing agent that only reduces iminium salt
It is: sodium triacetoxyborohydride!!
Preparation of Tertiary, Con’t
R
+
Na(MeCOO)3)3BH
NR'R"
R
NOTE: Secondary amine converts to
tertiary amine
R
NR'R"
H
R
tertiary amine
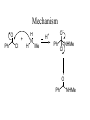
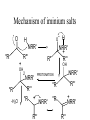
Mechanism of iminium salts
O
O
H
NRR'
R"'
"R
>
OH
"R
-H2O
2
NRR'
NRR'
+
R"'
R"
+
- H
OH
NRR'
PROTONATION
R"'
"R + ..
NRR'
R"'
"R
R"'
"R
+
NRR'
R"'