* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download C - b. finkel
Survey
Document related concepts
Transcript
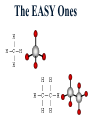
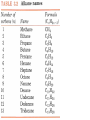
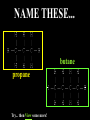
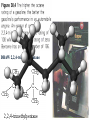
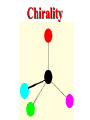
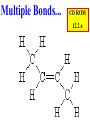
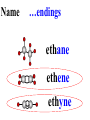
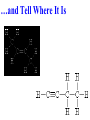
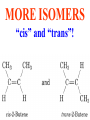
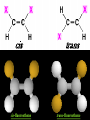
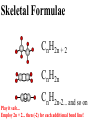
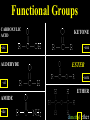
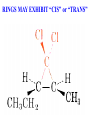
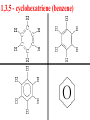
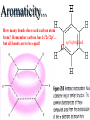
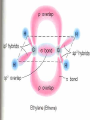
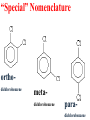
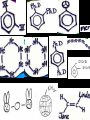
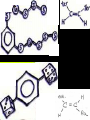
Honors Chemistry THICK BOOK Ch. 29 & 30 Organic Chemistry CD ROM 12 Intro. Links to the IUPAC PEOPLE WHY THE NAME “ORGANIC”? There are over six million organic compounds, both natural: foods, (carbohydrates, lipids, proteins, and vitamins) , furs, feathers, hides, skins, petroleum products and the organisms they came from... and synthetic: plastics, fibers, dyes, drugs, insecticides, herbicides, perfumes and flavoring agents. SO WHY “ORGANIC”? The name ORGANIC chemistry came from the word organism. Scientists thought some "vital force" that living organisms had was necessary to make an organic compound. In attempting to prepare ammonium cyanate from silver cyanide and ammonium chloride, a German chemist, Wöhler, accidentally synthesized urea in 1828. This synthesis of an ORGANIC substance from INORGANIC substances led to the disappearance of this vital force theory. “CARBON BASED LIFE FORMS” WHY CARBON? 4 VALENCE ELECTRONS 4 COVALENT BONDS CATENATION! CD ROM 12.1 a The EASY Ones NAME THESE... butane propane Try... then View some more! One Change… ...Changes Everything!!! Br 4 3 2 1 Br butane becomes bromobutane ACTUALLY... 2-bromobutane NOW… 2,2-dibromobutane VIEW organic reactions in progress !!! VARIATIONS... Branching ...like a crossword puzzle Multiple Bonds Substituents: (hetero-atoms and functional groups) Rings View Site with condensed notes. A BRANCH ON THE CHAIN... Two Branches… DRAW 2,2,4-trimethylpentane TRY: 3,5 - dimethyl - 4 - isopropyl octane C C C C-C-C-C-C-C-C-C C C ISOMERS CD ROM 12.1 b “isomers” Isomers : compounds with identical molecular composition but their structures are arranged differently. Isomerism : another reason why there are so many organic compounds. ISOMERS Formula C8H18 C10H22 C20H42 C40H82 Number of Isomers 18 75 366,319 6.25 x 1013 (approx.) ENANTIOMERS ARE THESE ISOMERS? ENANTIOMERS non-superimposable mirror images. Because of its tetrahedral bonding geometry, a C atom bonded to 4 different atoms or different structural groups can exist in 2 forms which are mirror images of one another and which cannot be superimposed upon one another. This is an important concept in biology because organisms usually contain (use or produce) only one of two possible enantiomers of a compound. Such molecules (and the C atom) are sometimes called chiral after the Greek word for hand; your left and right hands are the most common examples of nonsuperimposable mirror images. VIEW 5 MINUTE CLIP “CHEMISTRY ORGANIC Chirality” Chirality Multiple Bonds... CD ROM 12.2 a Name …endings ethane ethene ethyne …and Tell Where It Is MORE ISOMERS “cis” and “trans”! cis-fluoroethene trans-fluoroethene Skeletal Formulae CnH2n + 2 CnH2n CnH2n-2... and so on Play it safe... Employ 2n + 2... then (-2) for each additional bond line! Substituents... hetero-atoms 4 3 2 1 side chains Substituents... CD 12.3 “FUNCTIONAL GROUPS” and historic references Functional Groups alcohol Propanol or Propyl Alcohol Really should use an address… what is isopropyl alcohol? Functional Groups CARBOXYLIC ACID -oic ALDEHYDE KETONE -one ESTER -oate -al ETHER AMIDE -ide dimethyl ether ASSIGNMENT: RESEARCH AN ORGANIC COMPOUND CREATE A 3 SLIDE PRESENTATION http://en.wikipedia.org/wiki/List_of_organic_compounds SOME REALLY INTERESTING COMPOUNDS: For example… Sodium thiopental, better known as Sodium Pentothal Thiopental is still used in some places as a truth serum! For example… 2-Amino-3-(1H-indol-3-yl)propanoic acid , better known as Tryptophan Turkey meat and drowsiness! Novocaine 2-(diethylamino)ethyl 4-aminobenzoate functional gps: (2 aminos, ester, benzene ring) Mechanism: inhibits sodium influx through voltage gated sodium channels in the neuronal cell membrane of peripheral nerves action potential cannot arise signal conduction inhibited binds function of N-methyl-D-aspartate (NMDA) receptors as well as nicotinic acetylcholine receptors and the serotonin receptor-ion channel complex KNOWING NAMES... Antifreeze Ethylene glycol 1,2 - ethanediol Esters formed from the Rx between alcohols and acids. word 'ester' alone implies that the acid is an organic acid, but inorganic acids can also form esters. -ATP is a well-known phosphate ester in biology! -“halogenoalkanes” may be regarded as inorganic esters, of alcohols and hydrochloric acid. -We are focusing upon ORGANIC esters of organic acids RCOOR' where R can be hydrogen or an organic group: Esterification Esters in the food industry Esters are widely used for flavorings; many are 'nature-identical', that is synthetic versions of the esters present in the fruit. Fruit flavors are very complex, though, often arising from many different compounds, some of which are present in small quantities. Ethyl methanoate (ethyl formate): rum flavoring Propyl pentanoate (n-propyl n-valerate): pineapple flavoring Ethyl butanoate (ethyl butyrate): apple odor Octyl ethanoate (n-octyl acetate): orange odor Procedure Obtain two test tubes, 150 x 80 capacity; two one-holed rubber stoppers, #2; and two disposable Pasteur pipets, 2-mL vol, to act as air condensers. After deciding what two esters you would like to make, obtain the appropriate acids and alcohols (refer to reactants list). Formulations: propionic acid (1.8 mL) + ethanol (1.5 mL) butyric acid (1.0 mL) + isopentyl alcohol (1.5 mL) acetic acid (2.0 mL) + isopentyl alcohol (2.8 mL) acetic acid (2.0 mL) + n-butyl alcohol (2.8 mL) acetic acid (1.0 mL) + octyl alcohol (2.0 mL) salicylic acid (1.8 g) + methyl alcohol (1.0 mL) butyric acid (1.0 mL) + methyl alcohol (0.8 mL) trans-cinnamic acid (1.8 mL)+ ethyl alcohol (1.0 mL) LIKE A DOG CHASING ITS TAIL… CLEANING THINGS UP... MORE RINGS... RINGS MAY EXHIBIT “CIS” or “TRANS” 1,3,5 - cyclohexatriene (benzene) O Aromaticity... How many bonds does each carbon atom form? Remember carbon has 1s22s22p2… but all bonds are to be equal! C sp2 hybridized Nomenclature Hierarchy… IS THIS… pentyl benzene C-C-C-C-C- O O -C-C-C-C-C-C-C-C-C OR, IS THIS phenyl nonane “Special” Nomenclature orthodichlorobenzene metadichlorobenzene paradichlorobenzene “Special” Nomenclature “Xylene” with methyl’s ortho- meta- O para- Benzene Derivatives... Methylbenzene (Toluene) Chlorobenzene Hydroxybenzene (phenol) Benzoic Acid Aminobenzene (aniline)… where AMINES are R - NH2 O Fibers, films, and bottles made of polyester all contain… SOAP MAKING 7.4 a and b Video Clips CO KID C H SYL C H C H -O- H C VANIA