* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download File - need help with revision notes?
Survey
Document related concepts
Transcript
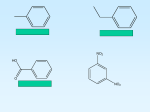
Arenes: Benzene Arenes are aromatic hydrocarbons containing 1 or more benzene rings. A benzene ring is a ring of 6 carbon atoms, each of which is also bonded to a hydrogen atom, with molecular formula C6H6. Benzene derivatives can be formed when one or more of the hydrogen atoms on a benzene ring is replaced by another atom or group of atoms. The Kekulé model of Benzene Nitrobenzene Bromobenzene Ethylbenzene In 1865, Kekulé proposed this structure of benzene, with alternate single and double bonds. Localised π bond formed by sideways overlap of 2 p orbitals above and below the plane of the ring. Benzene ring contains 3 localised π bonds: each contains 2 shared electrons between 2 carbon atoms. This model has high electron density Kekulé’s structure failed to explain the chemical and physical properties of benzene fully… Why was Kekulé wrong? Benzene does not undergo electrophilic addition. It will not decolourise bromine water . Enthalpy changes of hydrogenation is less exothermic than expected The Kekulé structure contained 3 double C=C bonds. When a double bond reacts with hydrogen, the enthalpy change is expected to be -120kJmol-1. So, for benzene, containing 3 double bonds, ΔH would be expected to be -360 kJmol-1. When benzene is hydrogenated however, ΔH is only -208 kJmol-1 . The actual structure of benzene is therefore more stable than the Kekulé structure. Bond lengths from X-Ray diffraction Kekulé structure of alternating single and double bonds should have 2 different bond lengths: shorter double bonds of 0.134nm length and longer single bonds of 0.153nm length. X ray diffraction however revealed that all six of the carbon-carbon bonds in benzene are the same length: 0.139nm – an intermediate between short C=C and long C-C bonds. Delocalised Model of Benzene Delocalised π bond is formed by sideways overlap of 6 p orbitals above and below the plane of the ring. The benzene ring contains 1 delocalised π bond which contains 6 electrons shared between 6 carbon atoms. It has a lower electron density than the Kekulé model. Each carbon atom in the ring has 4 outer shell electrons. 3 of these electrons bond to 2 other carbon atoms and a hydrogen atom. This leaves a 4th outer shell electron in a 2p orbital above and below the plane of the carbon atoms. The electron in the p orbital of the carbon atom overlaps with the electrons in the p orbitals of the carbon atoms on either side, resulting in a ring of electron density above and below the plane of the carbon atoms. Electrophilic Substitution Electrophilic substitution reactions occur in benzene chemistry because of the delocalised ring of electrons above and below the plane of the carbon atoms. 1. Two of the delocalised electrons are donated to the positive electrophile forming a covalent bond. 2. An intermediate forms that contains both the electrophile and the hydrogen atom that is being substituted. The delocalised π electron is disrupted and the intermediate is less stable than benzene. 3. C-H bond breaks and 2 electrons are returned to the delocalised ring. The unstable intermediate rapidly loses a hydrogen as a H+ ion. The delocalised ring is reformed and stability restored. Nitration of Benzene Benzene is nitrated to form nitrobenzene. Conditions: concentrated HNO3; concentrated H2SO4; 55° The electrophile in this reaction is the nitryl cation, NO2+. In order to generate this electrophile: HNO3 + H2SO4 H2NO3+ + HSO4H2NO3+ NO2+ + H2O Overall: HNO3 + H2SO4 NO2+ + H2O + HSO4- Reduction of nitrobenzene Nitrobenzene is reduced to form phenylamine. Conditions: concentrated HCl; Sn; reflux The manufacture of phenylamine therefore occurs in 2 steps: 1. Nitration of benzene to form nitrobenzene 2. Reduction of nitrobenzene to form phenylamine + 6[H] + 2H2O Manufacture of TNT (Trinitrotoluene) Methyl benzene reacts with nitric acid to form 2,4,6 trinitrotoluene and water. Halogenation of Benzene Electrophilic substitution with a halogen in the presence of a halogen carrier catalyst (FeBr3 or AlCl3) Benzene is unable to react with a halogen alone because the lower electron density of the delocalised benzene ring cannot induce a dipole on the halogen molecule. Bromination of Benzene Br2 / FeBr3 / anhydrous Generation of the Br+ electrophile using halogen carrier catalyst: Br2 + FeBr3 Br+ + FeBr4Regeneration of the halogen carrier catalyst: FeBr4- + H+ FeBr3 + HBr Chlorinationof Benzene Cl2 / AlCl3 / anhydrous Generation of the Cl+ electrophile using halogen carrier catalyst: Cl2 + AlCl3 Cl+ + AlCl4Regeneration of the halogen carrier catalyst: AlCl4- + H+ AlCl3 + HCl Comparing the reactivities of benzene and cyclohexene When bromine water is added to cyclohexene, a cyclic alkene, an Electrophilic addition reaction occurs. The π bond contains localised electrons between 2 carbon atoms. This is a region of high electron density, capable of inducing a dipole on the bromine molecule. Cyclohexene will decolourise bromine water form orange to colourless. When bromine water is added to benzene, there is no reaction The π bond in benzene is delocalised over 6 carbon atoms. It has a lower electron density than alkenes. The electron density is insufficient to induce a dipole on the bromine molecule. Bromine water will not decolourise: it will remain orange. A halogen carrier catalyst is required to generate the more powerful electrophile Br+ in order for a reaction to occur. Arenes: Phenol Phenol is hydroxybenzene. It is an aromatic alcohol – an OH hydroxyl group attached directly to a benzene ring. Solubility Phenol has low solubility The hydrophilic OH group can form hydrogen bonds with water Hydrophobic nonpolar benzene ring cannot form hydrogen bonds It is therefore partially soluble in water Bonding in Phenol Lone pair of electrons on oxygen atom is partially delocalised into the ring Increased electron density in the ring Phenol more able to induce dipole or attract electrophiles. Reaction of Phenol + H+ When dissolved in water, phenol forms a weak acidic solution by losing an H+ ion from the OH group. Reaction with sodium to form a phenoxide salt: Effervescence; sodium dissolves; phenol dissolves Reaction with sodium hydroxide to form a phenoxide salt: The phenol dissolves Comparing reactivities… Phenols react with Na and NaOH to form a phenoxide salt. Only reacts with Na, to form an alkoxide salt. Aliphatic Alcohols Carboxylic Acids Phenols (aromatic alcohols) Carboxylic acids are the most reactive; they react with Na, NaOH and Na2CO3 to form carboxylate salts. Halogenation of Phenol Phenol undergoes electrophilic substitution to form 2,4,6-tribromophenol Unlike benzene, this reaction will occur at room temperature without the need for a halogen carrier catalyst. Bromine will decolourise (orange to colourless) and a white precipitate of 2,4,6- tribromophenol forms. Phenol is more reactive than benzene because in phenol, the lone pair of electrons on oxygen is partially delocalised into the ring. Phenol has a higher electron density in the ring than benzene so phenol can induce a dipole in bromine, whereas benzene cannot (in the absence of a halogen carrier catalyst). Esterification of Phenols Ethanoic Anhydride + Phenol Phenyl Ethanoate + Ethanoic acid Conditions: Ethanoic Anhydride / methanol /warm Phenol is a weak nucleophile compared to non-aromatic alcohols because the lone pair on the oxygen is partially delocalised into the ring. Phenols do not react readily with carboxylic acids, so instead a more reactive acid anhydride is used. Uses of phenols: Detergents, antiseptics, disinfectants, preparation of aspirin and other pharmaceuticals, production of resins for paints, plastics. Comparing the relative reactivity of ethene, benzene and phenol towards Br2 Ethene Localised π bond: 2 electrons over 2 carbon atoms High electron density Can polarise (induce a dipole) in Br2 molecule Undergoes electrophilic addition reactions Benzene Delocalised π bond: 6 electrons over 6 carbon atoms Lower electron density than ethene Cannot polarise (induce a dipole) in Br2 molecule Undergoes electrophilic substitution reactions with a halogen carrier catalyst Phenol Delocalised π bond; lone pair of electrons on oxygen atom is partially dissociated into the ring Higher electron density in the ring than benzene Can polarise (induce a dipole) in Br2 molecule Undergoes electrophilic substitution reactions without a halogen carrier catalyst