* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Transition Metal Complexes
Hydroformylation wikipedia , lookup
Cluster chemistry wikipedia , lookup
Jahn–Teller effect wikipedia , lookup
Metal carbonyl wikipedia , lookup
Spin crossover wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Metalloprotein wikipedia , lookup
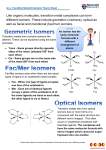
Transition Metal Complexes I The structures, nomenclature and isomers of coordination compounds Coordination Compounds Any compound containing a metal atom or ion with one or more ligands is called a coordination compound or complex. The ligands donate electrons to the metal via coordinate covalent bonds. Coordination Compounds The structures of these compounds was not always evident. Ions or molecules might be directly bonded to the metal, or serve as a counter ion for an ionic salt. [Mn(OH2)6] SO4 sulfate ion is outer sphere [Mn(OH2)5 SO4].H2O sulfate ion is inner sphere Coordination Compounds Early chemists approached transition metal complexes using the concept of “valences” adapted from main group metals. Metals with a +3 charge, such as iron(III) or cobalt(III) were believed to only make three bonds. A compound such as [Co(NH3)6]Cl3 was thought to have three Co-Cl bonds, with no way to explain the bonding of ammonia in the compound. Coordination Compounds One approach by Blomstrand proposed chains of linked ammonia molecules, with the nitrogens having five bonds and connecting a chloride to the metal. Alfred Werner proposed that the ammonia molecules could bond strongly and directly to the metal, with chlorides either directly bonded, or loosely bonded and ionic in solution. Coordination Compounds Coordination Compounds Jorgensen supported Blomstrand’s approach, and Werner, in order to support his theory, synthesized new compounds and studied their isomers. Eventually, in 1907, Werner prevailed and proved the octahedral geometry of coordination compounds. Coordination Compounds Alfred Werner (1866-1919) also determined the formulas and structures of many transition metal compounds by studying their isomers. Due to the existence of a variety of structural isomers, he proposed that complexes must have square planar and octahedral shapes. Possible Structures for 6Coordinate Cobalt Possible Structures for 6Coordinate Cobalt Possible Structures for 6Coordinate Cobalt Proposed Structures for 6Coordinate Cobalt Structure of Compounds Composition # ions Modern formulation PtCl2.4NH3 3 [Pt(NH3)4]2+ 2Cl- PtCl2.3NH3 2 [PtCl(NH3)3]+Cl- PtCl2.2NH3 0 [PtCl2 (NH3)2] (2 forms) Nomenclature of Coordination Compounds There is a separate system for naming coordination compounds: prefix indicating + ligand name + metal + (oxidation # # of ligands in roman numerals) or “ “ “ +“ “ “ + “ “ “ + (charge of complex) Nomenclature of Coordination Compounds 1. If ionic, the positive ion is named first, then the negative ion. 2. The inner coordination sphere is indicated by square brackets. In the formula, the metal is written first, followed by the ligands. In naming, the ligands are named first, then the metal. Nomenclature of Coordination Compounds 3. Prefixes: If the ligand itself contains a prefix in its name (ex. dimethyl amine), then the prefix to indicate the number of ligands changes, and the ligand name is placed in parenthesis. 2 di or bis 5 penta or pentakis 3 tri or tris 6 hexa or hexakis 4 tetra or tetrakis 7 hepta or heptakis 8 octa or octakis 9 nona or nonakis 10 deca or decakis Nomenclature of Coordination Compounds 4. Ligands are listed in alphabetical order (ignoring any prefixes). Most ligands have special names, with all negatively charged ligands ending in the letter“o”. Most neutral ligands retain their usual names, with the following common exceptions: NH3 ammine H2O aqua CO carbonyl Names of Common Ligands Formula BrCO32ClCNHOHO2- Name bromo carbonato chloro cyano hydrido hydroxo oxo Linkage Isomerism Formula NO2NO2- Name nitrito (via O) nitro (via N) Linkage Isomers Linkage isomers involve ligands that may bond via different sites. In this example, nitro bonds via nitrogen (NO2-), and nitrito bonds via an oxygen (NO2-). The compounds have different properties and colors. Polydentate Ligands Formula NH2CH2CH2NH2 Name ethylenediamine (en) Both amines of the ligand can attach at the metal forming a ring. Polydentate Ligands ethylenediaminetetraacetate (EDTA) EDTA is a hexadentate ligand. EDTA EDTA can wrap around a metal ion to coordinate at 6 (octahedral) sites. Ligands that bind to more than one site are called chelating agents. Nomenclature of Coordination Compounds 5. There are two systems for indicating the oxidation number of the metal. The more commonly used system indicates the oxidation number in Roman numerals in parentheses after the name of the metal. The other system puts the charge of the coordination complex in Arabic numbers in parentheses after the metal. [Cr(H2O)5Cl]2+ is pentaaquachlorochromium(III) or, pentaaquachlorochromium(2+). Nomenclature of Coordination Compounds 6. Using either system, if the transition metal complex is negative in charge, the name of the metal ends in ate. For example, [Pt(NH3)2Cl4]2- is named diamminetetrachloroplatinate(II). For metals with Latin names, the negatively charge complex uses: ferrate (for Fe) argentate (for Ag) plumbate (for Pb) stannate (for Sn) aurate (for Au) cuprate (for Cu) Nomenclature of Coordination Compounds 7. The complete name of the complex must also indicate the presence of geometric isomers. Prefixes such as cis, trans, mer, and fac are used to indicate the relative positions of similar ligands. In addition, stereoisomers are also possible with tetrahedral and octahedral geometries, and optical isomers are indicated with the prefixes ∆ and Λ. Nomenclature of Coordination Compounds 8. Bridging ligands between two metal atoms have the prefix μ. Isomerism Stereoisomerism Stereoisomers have the same connectivities but different spatial arrangements. In geometric isomers, the ligands have different spatial arrangements about the metal ion. Optical isomers are compounds with nonsuperimposable mirror images. Geometric Isomerism Geometric isomers differ in the geometric arrangement of the ligands around the central metal. Common examples are square planar complexes such as [Pt(NH3)2Cl2]. Geometric Isomerism In octahedral complexes, the prefixes cis and trans are used for complexes of the form [MX4Y2] Octahedral Complexes For complexes with the formula [MX3Y3], there are two spatial arrangements of the ligands. Octahedral Complexes fac stands for facial, and mer stands for meridian. Chirality Both four-coordinate and six-coordinate complexes exhibit chirality. Chiral molecules have either no symmetry elements (other than identity), or only a Cn axis. Tetrahedral complexes can be chiral in the same way that organic compounds are: they may have four different ligands. They may also have unsymmetrical chelating ligands. Chirality Square-planar complexes can also be chiral, as seen in these compounds of platinum(II) and palladium(II). Optical Isomers Octahedral complexes containing polydentate ligands can form optical isomers. Complexes with three rings, such as [Co(en)3]3+, can be viewed like a propeller with three blades. The structure can be either left or right handed, with non-superimposable mirror images. Optical Isomers The upper isomer is right handed, and the lower one is left handed. Optical Isomers Optical Isomers Optical Isomers Optical Isomers Optical Isomers Optical Isomers The right-handed isomer requires going clockwise to get from the upper triangle to the lower one. The prefix for this isomer is ∆. Optical Isomers The left-handed isomer requires going counterclockwise to get from the upper triangle to the lower one. The prefix for this isomer is Λ. Optical Isomers Octahedral complexes with two chelating ligands and two non-chelating ligands can also be optically active. Isomers Co(III) and ethylenediamine react to form several products. cis[CoCl2(en)2]+ is violet, and the trans isomer is green. The reaction also forms a yellow product, [Co(en)3]3+. Determine the number of isomers of each of the products. Label any enantiomers with the proper prefix (∆ or Λ). Isomer Problem The yellow product is [Co(en)3]+3. It exists as an enantiomeric pair. Isomer Problem The violet product consists of a pair of optical isomers. The green product is not optically active, as it has a mirror plane. Common Coordination Numbers & Structures Factors Affecting Coordination Number 1. The size of the central atom or ion. 2. Steric interactions between bulky ligands such as P(C6H5)3. 3. The electronic structure of the metal atom or ion. If the oxidation number is high, the metal can accept more electrons from the (Lewis base) ligands. Metals with many d electrons will have lower coordination numbers. Coordination Numbers Coordination numbers of 1, 2 and 3 are relatively rare. Coordination number 4 leads to two common structures: tetrahedral or square planar geometry. Square planar structures are most commonly seen with d8 metals such as Ni(II), Pd(II) or Pt(II), or when the ligand is planar. 5 Coordinate Complexes 5 coordinate complexes with monodentate ligands are often highly fluxional, so that axial and equatorial positions interchange. Once such method of interchange of these positions is via a Berry pseudorotation. Berry Pseudorotation A A A A A A 5 Coordinate Complexes A planar polydentate ligand, such as a porphyrin, may result in square pyramidal structures, when a fifth ligand is added, such as in myoglobin. 6-Coordinate Complexes 6-coordinate complexes are usually octahedral. Sometimes distortion from octahedral geometry occurs if a bidentate ligand has a small bite angle. 6-Coordinate Complexes In extreme cases, the “staggered” configuration of the octahedron can be distorted into a trigonal prism. 7- Coordinate Complexes These are more common for f-block elements, and include pentagonal bipyramids, capped octahedrons and capped trigonal prisms.