* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download I. Why Atoms Combine - Manchester High School
Oxidation state wikipedia , lookup
Molecular orbital diagram wikipedia , lookup
Metastable inner-shell molecular state wikipedia , lookup
Atomic theory wikipedia , lookup
Metallic bonding wikipedia , lookup
Hydrogen bond wikipedia , lookup
Electronegativity wikipedia , lookup
Bent's rule wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Molecular dynamics wikipedia , lookup
Metalloprotein wikipedia , lookup
Bond valence method wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Ionic compound wikipedia , lookup
History of molecular theory wikipedia , lookup
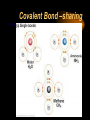
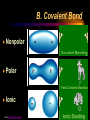
Chemical Bonds I. Why Atoms Combine Chemical Formula Chemical Bond Stability A. Chemical Formula Shows: 1) elements in the compound 2) ratio of their atoms H2O 1 oxygen atom 2 hydrogen atoms B. Chemical Bond Strong attractive force between atoms or ions in a molecule or compound. Formed by: • transferring e- (losing or gaining) • sharing e- C. Stability Octet Rule • most atoms form bonds in order to have 8 valence e• full outer energy level Ne • like the Noble Gases! Stability is the driving force behind bond formation! C. Stability Transferring e- Sharing e- Chemical Bonds II. Kinds of Chemical Bonds Ionic Bond Covalent Bond Comparison Chart A. Ionic Bond Attraction between 2 oppositely charged ions • Ions - charged atoms • formed by transferring efrom a metal to a nonmetal A. Ionic Bond • ions form a 3-D crystal lattice NaCl B. Covalent Bond Attraction between neutral atoms • formed by sharing e- between two nonmetals B. Covalent Bond • covalent bonds result in discrete molecules Cl2 NH3 H2O Covalent Bond –sharing B. Covalent Bond Nonpolar Covalent Bond • e- are shared equally • usually identical atoms B. Covalent Bond Polar Covalent Bond • e- are shared unequally between 2 different atoms • results in partial opposite charges + B. Covalent Bond Nonpolar Polar Ionic View Bonding Animations. C. Comparison Chart IONIC transferred from metal to nonmetal COVALENT shared between nonmetals Melting Point high low Soluble in Water yes usually not Electrons Conduct Electricity Other Properties yes no (solution or liquid) crystal lattice of ions, molecules, odorous liquids & gases crystalline solids http://bcs.whfreeman.com/thelifewi re/content/chp02/02020.html Chemical Bonds III. Naming Molecular Compounds Molecular Names Molecular Formulas A. Molecular Names Write the names of both elements. Change the final ending to -ide. Add prefixes to indicate subscripts. Only use mono- prefix with oxide. A. Molecular Names PREFIX monoditritetrapentahexa- SUBSCRIPT 1 2 3 4 5 6 A. Molecular Names CCl4 • carbon tetrachloride N2O • dinitrogen monoxide SF6 • sulfur hexafluoride B. Molecular Formulas Write the more metallic element first. Add subscripts according to prefixes. B. Molecular Formulas phosphorus trichloride • PCl3 dinitrogen pentoxide • N2O5 dihydrogen monoxide • H2O B. Molecular Formulas The Seven Diatomic Elements Br2 I2 N2 Cl2 H2 O2 F2 Chemical Bonds IV. Naming Ionic Compounds Oxidation Number Ionic Names Ionic Formulas A. Oxidation Number The charge on an ion. Indicates the # of e- gained/lost to become stable. 1+ 2+ 0 3+ 4+ 3- 2- 1- B. Ionic Names Write the names of both elements, cation first. Change the anion’s ending to -ide. Write the names of polyatomic ions. For ions with variable oxidation #’s, write the ox. # in parentheses using Roman numerals. Overall charge = 0. (Transition metals) B. Ionic Names NaBr • sodium bromide Na2CO3 • sodium carbonate FeCl3 • iron(III) chloride C. Ionic Formulas Write each ion. Put the cation first. Overall charge must equal zero. • If charges cancel, just write the symbols. • If not, crisscross the charges to find subscripts. Use parentheses when more than one polyatomic ion is needed. Roman numerals indicate the oxidation #. C. Ionic Formulas potassium chloride • K+ Cl- KCl magnesium nitrate • Mg2+ NO3- Mg(NO3)2 copper(II) chloride • Cu2+ Cl- CuCl2 C. Ionic Formulas calcium oxide • Ca2+ O2- CaO aluminum chlorate • Al3+ ClO3- Al(ClO3)3 iron(III) oxide • Fe3+ O2- Fe2O3 Covalent Bond Naming SiO2 silicon dioxide NO nitrogen monoxide XeF4 Xenon tetrafluoride Covalent Bond Naming P4S3 Tetraphosphorus trisulfide PBr3 phosphorus tribromide CS2 carbon disulfide Covalent bond formula Nitrogen trifluoride NF3 Dinitrogen pentoxide N2O5 Covalent Bond formula Trisilicon tetranitride Si3N4 Carbon dioxide CO2