* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Slide 1
Bond valence method wikipedia , lookup
Metal carbonyl wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Spin crossover wikipedia , lookup
Metalloprotein wikipedia , lookup
Oxidation state wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
A lack of synergy? An unusual
actinide-ligand bonding mode
Nik Kaltsoyannis
Department of Chemistry
University College London
What should I talk about?
“Anything you like, as long
as you are enthusiastic”
Outline of presentation
Part 1
•
•
A very brief introduction to actinide chemistry
The f elements by N Kaltsoyannis and P Scott, Oxford University Press (1999)
The Chemistry of the Actinide and Transactinide Elements, 3rd Edition, L. R.
Morss, N. Edelstein, and J. Fuger (eds), Springer (2006)
Part 2
Unusual metal-ligand bonding modes in molecular uranium complexes
Just checking…..
H
He
Li
Be
B
C
N
O
F
Ne
Na
Mg
Al
Si
P
S
Cl
Ar
K
Ca
Sc
Ti
V
Cr
Mn
Fe
Co
Ni
Cu
Zn
Ga
Ge
As
Se
Br
Kr
Rb
Sr
Y
Zr
Nb
Mo
Tc
Ru
Rh
Pd
Ag
Cd
In
Sn
Sb
Te
I
Xe
Cs
Ba
La
Hf
Ta
W
Re
Os
Ir
Pt
Au
Hg
Tl
Pb
Bi
Po
At
Rn
Fr
Ra
Ac
Rf
Db
Sg
Bh
Hs
Mt
Element 89
Ce
Pr
Nd
Pm
Sm
Eu
Gd
Tb
Dy
Ho
Er
Tm
Yb
Lu
Th
Pa
U
Np
Pu
Am
Cm
Bk
Cf
Es
Fm
Md
No
Lr
Element 90
Element 103
The ground electronic configurations of the actinides
Element
Thorium
Protactinium
Uranium
Neptunium
Plutonium
Americium
Curium
Berkelium
Californium
Einsteinium
Fermium
Mendelevium
Nobelium
Lawrencium
Electronic configuration
[Rn]6d27s2
[Rn]5f26d17s2
[Rn]5f36d17s2
[Rn]5f46d17s2
[Rn]5f67s2
[Rn]5f77s2
[Rn]5f76d17s2
[Rn]5f97s2
[Rn]5f107s2
[Rn]5f117s2
[Rn]5f127s2
[Rn]5f137s2
[Rn]5f147s2
[Rn]5f146d17s2
The shapes of the seven 5f orbitals
(cubic set).
5fy3, 5fx3, 5fz3
5fx(z2-y2), 5fy(z2-x2), 5fz(x2-y2)
5fxyz
The oxidation states adopted by the actinide elements in their compounds
+7
Formal Oxidation State
+6
+5
+4
+3
+2
Th
Pa
U
Np
Pu
Am
Cm
Bk
Cf
Es
Fm
Md
No
The most stable oxidation state in aqueous solution is represented by the
black circles. Open circles indicate other oxidation states adopted and
squares indicate that the oxidation state is found only in solids.
Lr
Radial distribution functions of selected atomic orbitals of U6+
(Enrique Batista, B3LYP, all-electron, 2nd order DK)
The particular challenges posed to quantum chemistry by the actinides
1
Lots of electrons.
2
Heavy elements relativistic effects are important (scalar - modification of
atomic orbital energies – and spin-orbit).
3
Large number of valence atomic orbitals of similar radial distribution and
energy (5f, 6p, 6d, 7s, 7p) actinide complexes are frequently open-shell,
with many closely-spaced electronic states. The correct description of electron
correlation effects is extremely important (and difficult) in these cases.
Part 2
Unusual metal-ligand bonding modes in molecular uranium complexes
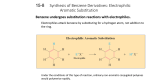
The classic Dewar-Chatt-Duncanson view of synergic bonding
s donation from filled
CO 3s orbital
p acceptance “backbonding”
into vacant CO 2p orbital
Qualitative MO
scheme for CO
Schematic view of synergic bonding
between CO and a transition metal
Qualitative MO scheme for octahedral
ML6 with p acceptor ligands (e.g. CO)
Are there CO complexes of the actinides?
Two views of (C5Me5)3U(CO)
Evans et al. JACS 125 (2003) 13831
[{(L)U}2(µ:h1,h1-CO)]
Meyer et al. JACS 127 (2005) 11242
“The hard, oxophilic f elements typically have a low binding affinity for the soft p bonding
CO ligand, and carbonyl complexes do not readily form”
f orbital to carbonyl 2p backbonding: the electronic
structures of (C5H5)3U(CO) and (C5H5)3U(OC)
B.E. Bursten and R.J. Strittmatter, JACS 109 (1987) 6606.
“Two major interactions of (C5H5)3U(CO) are discussed. The CO 3s
lone pair interacts primarily with the empty U 6d orbitals to form the
U-CO s bond, and extensive U 5f → CO 2p backbonding is observed”
R
R
R
N
N
N
N
N
U
U
N
N
N
N
R=SiMe2But
N
R
R
R
P. Roussel and P. Scott, JACS 120 (1998) 1070.
R
R
R
N
N
N
U
N
N
U
N
N
N
R
R
N
N
R
NH2
H2N
H3N
U
H2N
NH2
N
U
N
H2N
Back bonding without s bonding
N.Kaltsoyannis and P. Scott, Chem. Commun. (1998) 1665.
NH3
NH2
What is the oxidation state of the uranium atoms in [(C5Me5)2U]2(h-µ6:µ6-C6H6)?
Realistic possibilities include (a) U(II) and neutral benzene (b) U(III) and (benzene)2(most likely from experiment) and (c) U(IV) and (benzene)4-
How well does calculation reproduce the experimental geometry?
Interatomic distance/Å
Exp.
Calc.
U-U
4.396
4.406
U1-Cp* (av)
2.840
2.860
U2-Cp* (av)
2.830
2.840
C-C (benzene, complex)
1.440
1.440
C-C (benzene, free)
1.390
1.394
U1-C (benzene, av)
2.621
2.634
U1-C (benzene, max)
2.733
2.719
U1-C (benzene, min)
2.547
2.591
U2-C (benzene, av)
2.628
2.627
U2-C (benzene, max)
2.730
2.674
U2-C (benzene, min)
2.538
2.532
So why is the benzene ring so non-planar?
Hückel energies of the carbocyclic ring p orbitals
Calculation suggests
(a) each uranium gives up two electrons to the cp* ligands
(b) each uranium has two 5f-based electrons
(c) four electrons (two per uranium) are used to form a uranium/arene d bond
•The localisation properties of the four uranium/arene δ bonding electrons determine the
formal oxidation state of the metal centres.
•Population analysis indicates that these electrons have an approximately equal contribution
from both metal and arene, and hence the oxidation state of the uranium atoms is best
described as +3.
•The benzene ring is not neutral. Rather, it carries a charge close to -2, as there is transfer of
uranium 5f electron density into the benzene e2u C-C π* molecular orbitals. The benzene ring
is thus no longer Hückel aromatic, and is significantly non-planar as a result.
W.J. Evans, S.A. Kozimor, J. W. Ziller and N. Kaltsoyannis, JACS 126 (2004) 14533.
Arene-bridged diuranium complexes: inverted sandwiches supported by d backbonding
P.L. Diaconescu, P.L. Arnold, T.A. Baker, D.J. Mindiola
and C.C. Cummins, JACS 122 (2000) 6108.
(m-C7H8)[U(N[Ad]Ar)2]2
The two near degenerate d backbonding
orbitals of (m-C6H6)[U(NH2)2]2