* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Slide 1
Sulfuric acid wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Chemical bond wikipedia , lookup
Electrochemistry wikipedia , lookup
Ionic compound wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Surface properties of transition metal oxides wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
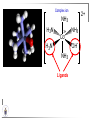
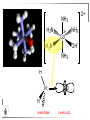
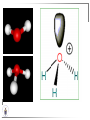
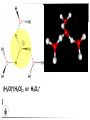
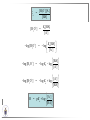
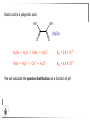
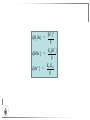
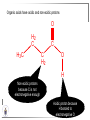
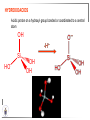
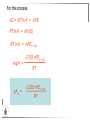
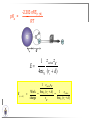
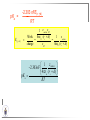
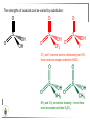
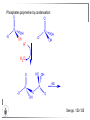
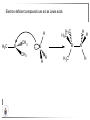
Chemistry II Inorganic Chemistry Part 2 Prof HM Marques C301 717-6737 [email protected] Chapters 4 and 5 Chapter 4 – Acids and Bases Revise from Chem I Bronsted-Lowry definition of an acid & a base Lewis definition of an acid & a base Complex ion 2+ NH3 H3N 3+ NH3 Co OH- H3 N NH3 Ligands 2+ NH3 H3N 3+ NH3 Co OH- H3 N NH3 H N H Co H Lewis base Lewis acid Water is amphiprotic (amphoteric) – it can act as either an acid or a base HCl(g) + H2O(l) H3O+ (aq) + Cl- (aq) Base NH3(g) + H2O(l) OH- (aq) + NH4+(aq) Acid (H3O)+(H2O)3 or H9O4+ HA(aq) + H2O (l) H3O+(aq) + A-(aq) Ka Acid dissociation constant [H3O+ ][A- ] = [HA] Measure of the strength of the acid pKa log ( Ka ) Acid strength decreases Perchloric acid (HClO4) Ka = 1010 pKa = -10 Sulphuric acid (H2SO4) 102 -2 Phosphoric (H3PO4) 7.5 x 10-3 1.92 Hydrocyanic acid (HCN) 4.9 x 10-10 9.31 The higher the pKa, the weaker the acid Ka [H3O+ ][A- ] = [HA] [H3O+ ] = K a [HA] [A- ] K [HA] log [H3O+ ] = log a - [A ] [HA] log [H3O+ ] = log K a log - [A ] [A- ] log [H3O ] = log Ka log [HA] + [A- ] pH = pKa log [HA] [A- ] pH = pKa log [HA] [Base] pH = pK a log [Acid] Henderson-Hasselbalch equation •When [Base] = [Acid], pH = pKa •At any pH, it can be shown (see Tut) that %[Base] = 100 1 10pKa pH %[Acid] = 100 1 10pH pKa B(aq) + H2O (l) OH-(aq) + HB+(aq) Kb Basicity constant [OH- ][HB+ ] = [B] Measure of the strength of the base pK b log ( K b ) The higher the pKb, the weaker the base H2O (l) + H2O (l) OH-(aq) + H3O+(aq) Kw Autoprotolysis constant of water = [OH - ][H 3O + ] = 1.00 x 10-14 at 25.0 oC Oxalic acid is a polyprotic acid: HO OH (H2Ox) O O H2Ox + H2O HOx- + H3O+ Ka1 = 5.9 x 10-2 HOx- + H2O Ox2- + H3O+ Ka2 = 6.4 X 10-5 We will calculate the species distribution as a function of pH K a1 Fractional abundance, so 0 x 1 = [HOx - ][H + ] [H 2Ox] K a2 = x(H 2 Ox) + x(HOx - ) + x(Ox 2- ) [Ox 2- ][H + ] [HOx - ] = 1 Ka2 [HOx - ] Ka1[H2Ox] x[H 2Ox] + x = 1 + x + + [H ] [H ] Ka1[H 2Ox] K a1 K a2 [H 2 Ox] x[H 2Ox] + x + x + + 2 [H ] [H ] Ka1 Ka1Ka2 x[H 2Ox] 1 + + + 2 [H ] [H ] = 1 = 1 Ka1 Ka1Ka2 x[H 2Ox] 1 + + + 2 [H ] [H ] [H+ ]2 Ka1[H + ] Ka1 Ka2 x[H 2Ox] + 2 [H ] x[H 2Ox] = [H + ]2 [H + ]2 K a1[H + ] K a1K a2 Similarly, K a1[H + ] x[HOx ] = β K a1 K a2 2x[Ox ] = β - = 1 = 1 [H + ]2 β [H + ]2 x[H 2 Ox] = β K a1[H + ] x[HOx ] = β K a1 K a2 2x[Ox ] = β - 1.0 HOx- 0.9 H2Ox Fractional abundance 0.8 Ox2- 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0.0 0.0 2.0 4.0 6.0 8.0 pH 10.0 12.0 14.0 Species distribution at pH 3 1.0 0.93 0.9 H2Ox HOx- Ox2- 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0.05 0.02 0.0 0.0 0.5 1.0 1.5 2.0 2.5 pH 93% HOx– ; 5% Ox2- ; 2% H2Ox 3.0 3.5 4.0 4.5 5.0 The acidity of a proton For a proton to be acidic it must be attached to an electronegative element (O, F, Cl, Br, I; to a lesser extent N, S) R––X––H If X is electronegative… …then the X-H bond can split heterolytically with X retaining the electron pair… R––X: + H+ …and delivering H+ to a Lewis base Organic acids have acidic and non-acidic protons O H2 C H3 C C C H2 O H Non-acidic protons because C is not electronegative enough Acidic proton because H bonded to electronegative O AQUA ACIDS Acidic proton on a water molecule coordinated to a metal ion If metal is able to polarise the M-O bond towards it… L L L M L O L H …that will cause the H-O bond to be polarised towards O… H …releasing H+ to be accepted by a Lewis base. AQUA ACIDS Acidic proton on a water molecule coordinated to a metal ion L L L L M L O L H L H L M L L O H + H+ Aqua acids are in principle polyprotic acids [Fe(OH2)6]3+ [Fe(OH2)5(OH)]2+ + H+ [Fe(OH2)4(OH)2]+ etc. + H+ HYDROXOACIDS Acidic proton on a hydroxyl group bonded or coordinated to a central atom OH Si HO OH OH OXOACIDS Acidic proton on a hydroxyl group bonded or coordinated to a central atom on which there is an oxo (=O) group Aqua acids, hydroxoacids and oxoacids may be successive stages of the deprotonation of an aqua acid OH OH2 L - 2 H+ L L Ru L L - H+ L Ru L OH2 O L Ru L OH L L L OH Aqua acids OH2 L Central atom in lower oxidation states L M L L OH2 s block, d block metals in lower (+1, +2, +3) oxidation states, metals on the left of the p block Strengths of aqua acids This can be rationalised using an electrostatic (ionic) model O H H H r+ radius of the metal ion of charge n+ d diameter of coordinated water molecule H+ Work done = [Potential at r = ] – [Potential at r = (r+ + d)] E 1 q1q2 4πε 0 r q1q2 1 0 4πε 0 (r d ) 1 zcation zH 4πε 0 (r d ) For this process, G = -RT ln K = -nFE RT ln K = nF(E) -RT ln K = -nFE(r -log K = pKa = +d) + -2.303 nFE(r +d) + RT -2.303 nFE(r RT ++d) pKa = -2.303 nFE(r RT E E( r d ) ++d) 1 zcation zH 4πε 0 (r d ) 1 zcation zH 4πε 0 (r d ) zcation Work 1 = = charge zH 4πε 0 (r d ) pKa = E( r d ) -2.303 nFE(r ++d) RT 1 zcation zH 4πε 0 (r d ) zcation Work 1 = = charge zH 4πε 0 (r d ) 1 zcation 2.303nF 4 o (r d ) pK a RT 1 zcation 2.303nF 4 o (r d ) pK a RT 2.303nFzcation 4 o (r d ) RT zcation -pK a (r ) pKa should become smaller, and acidity should increase with an increase in the charge on the ion a decrease of the size of the ion z -pK a ( r ) The term (z/r+) is also known as the ionic potential Alternatively we could say 2 z -pK a (r d ) by adding the charge on the proton and the diameter of water. The term (z2/(r+ + d)) is called the electrostatic parameter pKa gets smaller and acidity increases How good is this electrostatic model (for gas phase) in solution? as electrostatic parameter increases... See Fig. 4.3 How good is this electrostatic model (for gas phase) in solution? Model quite poor for many of the d block ions; their acidity is often much higher than predicted by the model Model quite good for s block ions, some d block ions, and the lanthanides Major reason for failure of the model: bonding between the metal and its ligands often not purely ionic, and there is some covalency in metalligand bonds. Model is worst for metals like Sn2+ and Hg2+ that form very covalent complexes. L L The more covalent the M-O bond… …the more the O-H bond is polarised towards O… L M L O L H H …the more readily H+ is lost, and the more acidic the compound Oxoacids Formed by electronegative elements top right of periodic table (e.g., N, P, S, Cl) elements in high oxidation state (e.g., Te, I, As, Se) See Table 4.2 General formula: OpE(OH)q H3PO3, phosphorus acid, is O1(PH)(OH)2 Pauling’s rules Empirical rules that allow one to estimate the pKa of oxoacids General formula: OpE(OH)q 1. pKa 8 – 5p 2. For p > 1, each successive pKa increases by about 5 units O Example Estimate the pKas of H3AsO4 As HO Actual values: 2.3, 6.9, 11.5 *Estimates are usually good to within 1-2 units OH OH The strengths of oxoacids can be varied by substitution: O O O O S S S OH OH O OH O CF3 OH F CF3 and F are more electron withdrawing than OH; these acids are stronger acids than H2SO4 The strengths of oxoacids can be varied by substitution: O O O S S OH OH O ...polarisesO the OH bond, making the proton more acidic OH O CF3 Electron withdrawl... S OH F The strengths of oxoacids can be varied by substitution: O O O O S S S OH OH O OH O CF3 OH F CF3 and F are more electron withdrawing than OH; these acids are stronger acids than H2SO4 NH2 and CH3 are electron donating – hence these acids are weaker acid than H2SO4 Oxides Oxides of non-metals are acidic. When dissolved in water, they bind water and release a proton SO3(g) + H2O(l) → H2SO4(aq) → H+(aq) + HSO4–(aq) O O + H2O S O O S O SO3 is the anhydride of H2SO4 OH OH Acidic oxides are neutralised by bases SO2 + NaOH → Na+HSO3– Oxides of metals are basic. When dissolved in water, they accept a proton from water, producing an alkaline solution MgO(s) + H2O(l) → Mg(OH)2(s) Mg2+(aq) + 2OH–(aq) Acidic and basic oxides neutralised each other CaO + SO2 → CaSO3 Oxides of the elements in the boundary region between metals and nonmetals often show amphoteric behaviour. Fig. 4.4 In the d block, metals in low oxidation states tend to be basic; amphoteric in their intermediate oxidation states; and acidic in high oxidation states Fig. 4.5 Amphoteric axides will react with acids and bases… Ga2O3 + 6H3O+ + 3H2O → 2[Ga(H2O)6]3+ Ga2O3 + 2OH– + 3H2O → 2[Ga(OH)4]– Polymerisation of aqua ions Aqua ions of metals that have basic or amphoteric oxides polymerise and precipitate as pH is increased. [Fe(OH2)6]3+ 3+ OH2 H2O - exists in strongly acidic solution OH2 increase pH H2O OH2 OH2 OH2 Fe Fe H2O 2+ OH2 H2O OH OH2 [Fe(H2O)6]3+(aq) + (3+n)H2O(l) → Fe(OH)3•nH2O(s) + 3H3O+(aq) polymer of Fe(OH)3 Similarly: [Al(H2O)6]3+(aq) + (3+n)H2O(l) → Al(OH)3•nH2O(s) + 3H3O+(aq) As pH is increased further, the species redissolve because both Al(OH)3 and Fe(OH)3 are amphoteric. Al(OH)3 + OH– → [Al(OH)4]– Polyoxyanions With early 3d elements, or oxides of elements in high oxidation states eg V2O5 eg PO43As base is added, condensation reactions occur, and polyoxyanions are formed VO42- V2O72- V3O93- [H2V10O28]4- Phosphates polymerise by condensation: H+ H2 O etc See pp. 123-125 Lewis Acids and Bases We have seen that compounds such as [Co(NH3)6]3+ are complexes between ligands (Lewis bases) and a metal (Lewis acid) Electron deficient compounds can act as Lewis acids H H3C B CH3 H H3C H3C B N CH3 H H H3C H N H Self-study: Write brief notes on the Lewis acid properties of compounds of the elements of the s block, of Group 13, 14, 15, 16 and the halogens (p. 126-128) Hard and Soft Acids and Bases (HSAB) R.G. Pearson Let A be a Lewis acid, and B a base Measure log K for the reaction A + B AB If for B = halide, the order of log K is I– < Br– < Cl– < F– then A is called a hard acid If for B = halide, the order of log K is I– > Br– > Cl– > F– then A is called a soft acid Fig. 4.10 Al3+ is a hard Lewis acid Log K increases with the ionic potential = z/r Anion– Al3+ r ionic radius increases F 1 zmetal zanion 4πε o r2 Strength of complex 1/r2 bonding is largely ionic Hg2+ is a soft Lewis acid Log K increases as the radius of the anion increases Hg2+ Anion– Hg2+ Anion– ionic radius increases Strength of complex increases as overlap between orbitals of the anion sand orbitals of the metal increases with increasing size of anion bonding is largely covalent Similarly, if we take a hard metal ion (like Al3+), then bases which bind strongly to it are hard Lewis bases; bases which do not bind strongly are soft Lewis bases. Pearson’s Principle: Hard Lewis acids prefer to bind to hard Lewis bases; soft Lewis acids prefer to bind to soft Lewis bases See Table 5.3 for a listing of hard and soft Lewis acids and bases In summary: Hard metal ions are small, usually highly charged. Their electron cloud is not readily polarisable. • Alkali metals (Li+, Na+, … ) • Alkali earth metal (Mg2+, Ca2+, … ) • H+ • Lighter transition metals in their higher oxidation states: Ti(IV), Ti(III), Cr(III), Fe(III), Co(III) … Hard Bases: contain the smaller electronegative atoms, especially O, N, F and Cl. These donor atoms also have rather unpolarisable electron clouds Soft metal ions are larger metal ions, often in their lower oxidation states. Their electron cloud is readily polarisable. • Heavier transition metals (Pt, Rh, Ir) • Transition metals in their lower oxidation state Cu(I), Ag(I), Hg(I), Hg(II), Pd(II), Pt(I), Pt(II) • As we move across the d block the +2 oxidation state is stabilised – i.e., get Sc(III), not Sc(II); but Zn(II) not Zn(III). Hence softness tends to increase across the d block, and down each group Soft Bases: contain the larger, more polarisable and less electronegative atoms, especially S, Se, P, C and As. Common soft bases include: • H- (hydride) • C donors • S, P, As donors • I- Example Arrange the following ligands in the order of increasing log K for binding to Fe(III) and to Pb(II) (donor atoms underlined) (a) (b) (c) (d) CH3SCH3 CH3OCH3 CH3SCH3O- Example Explain why Cu(I) and Cu(II) are found in nature as the sulphide (CuS, Cu2S) but Ti(IV) and Fe(III) are found as their oxides (TiO2, Fe2O3)