* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Bidentate & multidentate ligands File
Jahn–Teller effect wikipedia , lookup
Cluster chemistry wikipedia , lookup
Hydroformylation wikipedia , lookup
Metal carbonyl wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Spin crossover wikipedia , lookup
Metalloprotein wikipedia , lookup
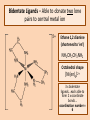
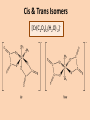
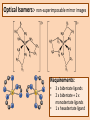
Bidentate & Multidentate Ligands -Explain and use the term bidentate ligand - Describe stereoisomerism in transition element multidentate complexes (cis-trans & optical isomerism) Bidentate Ligands – Able to donate two lone pairs to central metal ion Ethane 1,2 diamine (shortened to ‘en’) NH2CH2CH2NH2 Octahedral shape [Ni(en)3]2+ 3 x bidentate ligands...each able to form 2 x coordinate bonds... coordination number = 6 Cis & Trans Isomers [Cr(C2O4)2(H2O) 2]- Hexadentate Ligand - EDTA 6 lone pairs to use to form coordinate bonds Optical Isomers:- non-superimposable mirror images Requirements: • • • 3 x bidentate ligands 2 x bidentate + 2 x monodentate ligands 1 x hexadentate ligand Summary Question • Iron (III) can form the complex ion [Fe(C2O4)3]3with three ethanedioate ions. The ethanedioate ion is a bidentate a) Explain the term “bidentate ligand”. b) What is the coordination number of the [Fe(C2O4)3]3complex. c) Use your answer to part (b) to suggest what shape the [Fe(C2O4)3]3- complex is and draw it.