* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download T 2g
Survey
Document related concepts
Transcript
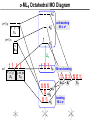
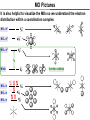
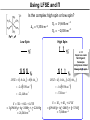
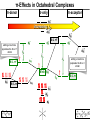
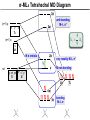
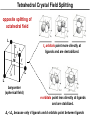
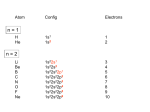
Coordination Chemistry II: Ligand Field Theory Chapter 10 Friday, November 22, 2013 Ligand Field Theory In LFT we use metal valence orbitals and ligand frontier orbitals to make metal–ligand molecular orbitals Metal valence orbitals: Sc – Zn 3d 4s 4p 4d 5s 5p Y – Cd Ligand frontier orbital: La – Hg 5d 6s 6p Oh first! We already did this: see 10/18 lecture. For now we will only consider σ-bonding with the ligands σ-MOs for Octahedral Complexes We use group theory to understand how metal and σ-ligand orbitals interact in a complex: nd (n+1)s z2 x2–y2 xy xz yz Eg T2g s A1g (n+1)p x y z T1u Oh symmetries (irr. reps.) of the metal valence orbitals are obtained directly from the character table We need to determine the reducible representation for the σ-ligand orbitals in Oh: C2′ C4 S4 C2 σh Γσ the total ligand representation (Γ6L) can be decomposed as we learned in 4.4.2 E 8C3 6C2 6C4 3C2′ i 6S4 8S6 3σh 6σd irreducible representations 6 0 0 2 2 0 0 0 4 2 A1g + Eg + T1u σ-ML6 Octahedral MO Diagram t1u* (n+1)p x y z T1u a1g* (n+1)s anti-bonding M–L σ* eg* s A1g Δo nd z2 x2–y2 xy xz yz Eg T2g t2g M non-bonding A1g t1u a1g eg bonding M–L σ Eg T1u MO Pictures It is also helpful to visualize the MOs so we understand the electron distribution within a coordination complex M–L σ* t1u* M–L σ* a1g* M–L σ* eg* ∆O M nb t2g M–L σ t1u M–L σ M–L σ a1g eg frontier orbitals Adding Metal Electrons t1u* a1g* eg* t2g t1u a1g eg Metal ions typically have some valence electrons that can be accommodated in the metal d orbitals • d0 ions – Ti4+, Zr4+, V5+, Ta5+, Cr6+, Mo6+, etc. • d1 ions – Ti3+, V4+, Ta4+, Cr5+, Mo5+, etc. • d2 ions – V3+, Ta3+, Cr4+, Mo4+, etc. • d3 ions – V2+, Ta2+, Cr3+, Mo3+, Mn4+, etc. • d4-d7 – hold on • d8 ions – Co1+, Ni2+, Cu3+, etc. • d9 ions – Ni1+, Cu2+, etc. • d10 ions – Cu1+, Zn2+, etc. High Spin and Low Spin t1u* a1g* a1g* •d4 ions – Cr2+, Mo2+, Mn3+, Fe4+, Ru4+, etc. eg* •d5 ions – Mn2+, Re2+, Fe3+, Ru3+, etc. •d6 ions – Fe2+, Ru2+, Co3+, Rh3+, Pt4+, etc. t2g t2g •d7 a1g eg For ions – Fe1+, Ru1+, Co2+, Rh2+, Ni3+, etc. d4-d7 electron counts: • when ∆o > Πtotal ➙ low spin • when ∆o < Πtotal ➙ high spin t1u a1g eg LOW SPIN HIGH SPIN eg* t1u t1u* The situation is a little more complicated for d4-d7 metals: High Spin and Low Spin Electron configurations for octahedral complexes, e.g. [M(H2O)6]+n. Only the d4 through d7 cases can be either high-spin or low spin. Δ<Π Δ>Π Weak-field ligands: - Small Δ, High spin complexes Strong-field ligands: - Large Δ, Low spin complexes Electron Pairing Energy The total electron pairing energy, Πtotal, has two components, Πc and Πe • Πc is a destabilizing energy for the Coulombic repulsion associated with putting two electrons into the same orbital • Πe is a stabilizing energy for electron exchange associated with two electrons having parallel spin d4 HS d8 eg* d6 LS eg* eg* Πe only counts for electrons at the same energy! t2g t2g t2g total 3e 0c total 7e 3c total 6e 3c LFSE 30.4 O 10.6 O 0.6 O LFSE 6 0.4 O 2 0.6 O 1.2 O LFSE 6 0.4 O 0 0.6 O 2.4 O Using LFSE and Π Is the complex high spin or low spin? Δ𝑜 = 9,350𝑐𝑚−1 Π𝑐 = 19,600𝑐𝑚−1 Π𝑒 = −2,000𝑐𝑚−1 Fe2+, d6 Low Spin High Spin eg* eg* t2g LFSE 6 0.4 O 0 0.6 O 2.4 9350cm 1 22, 440cm 1 𝐸 = 3Π𝑐 + 6Π𝑒 + 𝐿𝐹𝑆𝐸 = 3 19600 + 6 −2000 + −22400 = 24,360𝑐𝑚−1 Δ<Π Aqua is a weak field ligand; hexaaqua complexes almost always high spin t2g LFSE 4 0.4 O 2 0.6 O 0.4 9350cm 1 3740cm 1 𝐸 = 1Π𝑐 + 4Π𝑒 + 𝐿𝐹𝑆𝐸 = 19600 + 4 −2000 + −3740 = 7,860𝑐𝑚−1 π-MOs for Octahedral Complexes The reducible representation for the π-ligand orbitals in Oh: x and y axes on each ligand Γπ E 8C3 6C2 6C4 3C2′ i 6S4 8S6 3σh 6σd irreducible representations 12 0 0 0 -4 0 0 0 0 0 T1g + T2g + T1u + T2u The non-bonding t2g orbitals of an octahedral metal complex are oriented perfectly to form π-bonds with ligands π Donor vs π Acceptor Ligands The nature of the metal ligand π interaction is dependent on the type of ligand. • π-donor ligands are ligands with one or more lone pairs of electrons in p orbitals on the donor atom that can donate to empty orbitals on the metal. • preferred for metals with high oxidation states and low d electron count (d0-d3) Examples: Cl–, Br–, I–, OR–, SR–, NR2–, O2–, NR2–, N3– • π-acceptor ligands (π-acidic ligands) are ligands with empty π* orbitals on the donor atom that can accept electrons from the metal. • preferred for metals with low oxidation states and high d electron count (d6 or higher) • donation of electron density from the metal to the ligand π* orbital results in weakening of the multiple ligand bond Examples: CO, NO, CN-, pyridine “π back bonding” π-Effects in Octahedral Complexes σ-only π-donor π-acceptor t1u* increasing ∆O a1g* adding d electrons populates the M–L π* orbital M–L π* eg* eg* t2g* M–L π* t2g* ∆o ∆o ∆o t2g* M nb t2g t2g t2g M–L π eg* t2g t1u a1g eg adding d electrons populates the M–L π orbital M–L π π-Effects in Octahedral Complexes strong field, low spin weak field, high spin Spectrochemical Series The trend in ∆O that arises from π-donor, σ-only, and π-acceptor ligands is the basis for the Spectrochemical Series. For [ML6]n+ complexes: increasing ∆O I– < Br– < Cl– < OH– < RCO2– < F– < H2O < NCS– < NH3 < en < NO2– < phen < CN– ≅ CO π donor ligands σ only ligands π acceptor ligands • • weak-field ligands high-spin complexes for 3d metals* • • strong-field ligands low-spin complexes for 3d metals* The value of Δo also depends systematically on the metal: 1. Δo increases with increasing oxidation number. 2. Δo increases down a group. → both trends are due to stronger metal-ligand bonding. * Due to effect #2, octahedral 3d metal complexes can be low spin or high spin, but 4d and 5d metal complexes are always low spin. σ-MOs for Tetrahedral Complexes Four-coordinate tetrahedral complexes are ubiquitous throughout the transition metals. nd (n+1)s z2 x2–y2 xy xz yz E T2 s A1 (n+1)p x y T2 z the irr. reps. of the metal valence orbitals are obtained directly from the character table Td For the ligand orbitals we need to consider how the Lewis base pairs transform in the Td point group. The result is: Γσ = A1 + T2 A1 T2 σ-ML4 Tetrahedral MO Diagram 3t2 (n+1)p x y T2 2a1 z 4 t o 9 (n+1)s s A1 M d orbitals 2t2 very weakly M–L σ* ∆t e nd xy xz yz T2 M non-bonding z2 x2–y2 E A1 1a1 1t2 bonding M–L σ T2 Tetrahedral Complexes • Metal d orbitals are split into a non-bonding E set and a very weakly anti-bond T2 set • tetrahedral geometry can accommodate all d electron counts, from d0 to d10 eg* very weakly M–L σ* t2 * strongly M–L σ* ∆o ∆t M non-bonding e t2g M non-bonding 4 t o 9 • Δt is small compared to Δo: • All tetrahedral complexes of the 3d transition metals are HIGH SPIN! • Tetrahedral complexes of the heavier transition metals are low spin. Tetrahedral Crystal Field Splitting opposite splitting of octahedral field L z M t2 orbitals point more directly at ligands and are destabilized. L y x L L barycenter (spherical field) e orbitals point less directly at ligands and are stabilized. Δt < Δo because only 4 ligands and d orbitals point between ligands