* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Effect of pH on Hemoglobin Oxygen Affinity H + = O 2 Affinity In
Photosynthesis wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Biochemistry wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Homeostasis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
High-altitude adaptation in humans wikipedia , lookup
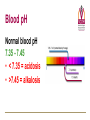
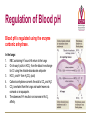
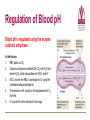
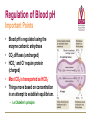
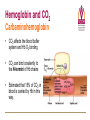
Transport of Oxygen and Carbon Dioxide Matthew L. Fowler, Ph.D. Cell Biology and Physiology Block 4 Reading • Guyton Chapters 39 and 40 • Important – pp. 490-492 Learning Objectives • Compare and contrast myoglobin to hemoglobin and explain how myoglobin can damage the kidney • Describe the barriers that oxygen needs to cross to enter tissue and how this occurs • Draw and explain the oxygen binding curve for hemoglobin and myoglobin including the hyperbolic curve for myoglobin, the sigmoidal curve for hemoglobin, the difference in oxygen affinity of the two proteins, and why these affinities are appropriate to meet oxygen needs in working muscle versus resting muscle. • List and explain the factors that modify hemoglobin oxygen binding affinity. • List and provide a brief description of the different forms of hemoglobin including Hb F, HbA, thalasemmia, methemoglobin, carbaminohemoglobin, and sickle cell and detail how these effect hemoglobin and blood flow. • Explain how, temperature, pH, CO2 and 2,3 BPG affect oxygen binding to Hb. • Define oxygen partial pressure (tension), oxygen content, and percent hemoglobin saturation as they pertain to blood. • Detail how oxygen, bicarbonate and CO2 are transported in the blood and the Bohr and Haldane effect • Explain the basis for how carbonic anhydrase effects hemoglobin and pH. Overview • • • • Oxygen is necessary for metabolism O2 must be captured as a gas, transferred to a liquid environment for transport. CO2 from a liquid environment must be transported to the lungs and returned to the atmosphere as a gas. Changes required to achieve this: 1. Pressure • 2. Concentration • • 3. Free gases exert partial pressures based on their % composition Attempt at equilibrium is a driving force Gases diffuse (smaller gases more easily); down a concentration gradient Solubility • • Bound gases no longer exert a pressure CO2 is much more soluble than O2 Red Blood Cells • 4-6 x 106 cells/μL • Hb in cytoplasm – ~250 million molecules/cell – Transports O2… and CO2… sometimes bad things… more later… • Complex ECM – Sialic acid residues provide negative charge • Prevent sticking and rolleaux (stacking) • Extracellular branches of membrane glycolipids define blood type RBC Embryology • First couple weeks – Yolk sac • Middle trimester – Fetal liver (also spleen/lymph nodes) • Last month – Bone marrow • After puberty only from rib, sternum, and vertebra Clinical Correlate: Ectopic production or RBC’s can occur in renal cell carcinoma and hepatocellular carcinoma Transport of Oxygen Hemoglobin • O2 and CO2 transport protein • Blood chemistry affects hemoglobin oxygen binding properties…key to function. • Used to transport O2 due to low solubility • O2 binds to Hb at high PO2 levels • Binding – up to 4 O2 Transport of Oxygen Hemoglobin Various Subunits During Development • Always have 2 α-chains • Fetal Hemoglobin (HbF) – α2γ2 (primary) • Post-Birth Hemoglobin – HbA = α2β2 – HbA2 = α2δ2 Sickle Cell Anemia Hemoglobin • • • • • • A single amino acid change (Val6Glu) in the Hb β chain (Hb S) changes shape of Hb Causes Hb (S) to polymerize with Hb (A) and form large insoluble aggregates (clumps) – Exacerbated by low pH Insoluble Hb has less affinity for O2 Hb insolubility results in sickling of cells Sickled cells act as thrombi in capillaries Result is ischemia manifesting as pain and/or hemolytic crisis – RBC lysis • Hb levels • Indirect bilirubin levels • Reticulocytes • LDH Abnormal States Methemoglobin (MetHb) • • • • • • Fe3+ (ferric) state = abnormal (methemoglobin) Fe2+ (ferrous) = normal hemoglobin 1% in normal person Methemoglobin cannot carry oxygen Blood with high concentrations of metHb is bluishchocolate-brown in color Inorganic nitrites promote ferrous (Fe2+) to ferric (Fe3+) NADH-dependent methemoglobin reductase MetHb Hb • Tx. Methylene Blue Cyanide (CN) Treatment Methemoglobin (MetHb) • Cyanide inhibits cytochrome c oxidase – • Final step in the electron transport chain Amyl nitrite used to treat CN poisoning – Converts Hb to MetHb Treatment Mechanism 1. 2. 3. MetHb binds (CN) CN-MetHb = Cyanomethemoglobin (non-toxic) Cyanomethemoglobin metabolically eliminated Carbon monoxide (CO) Toxicity Carboxyhemoglobin (COHb) CO has a higher affinity for Hb than O2 or CO2 – – Binding of CO to Hb forms carboxyhemoglobin (COHb) Shifts curve to the left • Eventually results in hyperbolic curve Treatment Mechanism 1. 2. 3. Remove from CO and supplement O2 Higher O2 than CO allows O2 to out-compete CO for hemoglobin sites and increases dissociation of CO HCO3- can be administered if for concurrent metabolic acidosis Storage of Oxygen Myoglobin • Stores oxygen in muscle for emergency use – Reserve for O2 • Higher in slow twitch muscles • Pathological - not normally in circulation (lysis of cells) can lead to kidney damage • Binding - 1 O2 Rhabdomyolysis Myoglobin Breakdown of muscle fibers that Rhabdomyolysis and Renal Failure from lysed muscle cells circulated in blood (myoglobinuria). leads to the release of myoglobin 1.2. Myoglobin Myoglobin interacts with Tamm-Horsfall protein in the nephron . 3. Casts (solid aggregates) obstruct the normal flow of fluid. into the bloodstream. 4. High levels of uric acid and acidification of the filtrate increase cast formation. 5. Under conditions of hypotension, decreased renal perfusion leads to uric • Results in acute renal failure acid crystal formation in the renal tubules, resulting in obstruction. 6. Iron released from the heme generates ROS that damage the kidney cells. • Sensitive marker for muscle 7. These processes lead to acute tubular necrosis, the destruction of the cells of tubules. injury – Statins – Severe burns – Myocardial infarction • Though a poor indicator for MI Result Glomerular filtration rate falls and the kidney is unable to perform its normal excretory functions. Outcome Disruption of electrolyte regulation, hyperkalemia, and interference with vitamin D metabolism resulting in hypocalcemia. Hemoglobin has Cooperative Binding of O2 O2 is never fully unloaded from Hb Random Sample • PO2 of 27 mmHg = 50% O2 Saturation Normal venous blood • PO2 of 40 mm Hg = 75% O2 Saturation Normal Arterial Blood • PO2 of 100 mm Hg = 96% O2 Saturation Factors that Modify Hemoglobin Oxygen Binding Affinity • • • • • Blood pH CO2 levels 2,3-bisphosphoglycerate Temperature Hb chain composition – HbF has higher affinity for O2 • Allows fetus to sequester more oxygen from the mother Blood pH Normal blood pH 7.35 - 7.45 • < 7.35 = acidosis • >7.45 = alkalosis Regulation of Blood pH Blood pH is regulated using the enzyme carbonic anhydrase. In the lungs: 1. RBC containing H+ bound Hb return to the lungs 2. On its way it pulls in HCO3- from the blood in exchange for Cl- using the chloride-bicarbonate antiporter 3. HCO3- and H+ form H2CO3 (acid) 4. Carbonic anhydrase converts the acid to CO2 and H2O 5. CO2 is exhaled from the lungs and water leaves via osmosis or an aquaporin. 6. The absence of H+ results in an increase in Hb O2 affinity. Regulation of Blood pH Blood pH is regulated using the enzyme carbonic anhydrase. In the tissues: 1. RBC picks up CO2 2. Carbonic anhydrase combines the CO2 with H2O and forms H2CO3 which dissociates into HCO3- and H+ 3. HCO3- leaves the RBC in exchange for Cl- using the chloride-bicarbonate antiporter 4. The presence of H+ assists in the displacement of O2 from Hb 5. H+ bound Hb is then returned to the lungs. Regulation of Blood pH Important Points • Blood pH is regulated using the enzyme carbonic anhydrase • CO2 diffuses (uncharged) • HCO3- and Cl- require protein (charged) • Most CO2 is transported as HCO3• Things move based on concentration in an attempt to establish equilibrium. – Le Chatelier's principle The Bohr Effect CO2 acts as a weak acid in the presence of H2O resulting in the production of H+ ions shifting the Hb Saturation Curve to the right. How? Carbonic anhydrase Hemoglobin as a Buffer • O2 and H+ are competing for the same site on Hb • During exercise you produce lots of CO2 • CO2+ H2O HCO3- + H+ • H+ = O2 Affinity = O2 unloading from Hb – • Affinity – a protein’s ability to capture a substrate Since Hb can bind H+ it can act as a physiological buffer Hemoglobin and CO2 Carbaminohemoglobin • CO2 affects the blood buffer system and Hb O2 binding • CO2 can bind covalently to the N-termini of Hb chains • Estimated that 15% of CO2 in blood is carried by Hb in this way. The Haldane Effect Binding of O2 to Hb reduces Hb CO2 affinity • Deoxygenation of the blood increases its ability to carry carbon dioxide • Conversely, oxygenated blood has a reduced capacity for carbon dioxide. Effect of pH on Hemoglobin Oxygen Affinity H+ = O2 Affinity In the muscles/tissues: 1. Metabolism = H+ (pH) 2. H+ (pH) = O2 requirements 3. H+ (pH) = O2 affinity 4. O2 affinity = O2 binding to Hb 5. O2 binding to Hb = O2 for tissues Take Home: Optimized for O2 release Effect of pH on Hemoglobin Oxygen Affinity H+ = O2 Affinity In the lungs: 1. Exhalation = CO2 2. CO2 = H+ (pH) 3. H+ (pH) = O2 affinity 4. O2 affinity = O2 binding to Hb Take Home: Optimized for O2 binding Effect of pH on Myoglobin Oxygen Affinity No Effect!!! • • Myoglobin follows hyperbolic saturation Not a cooperative-binding system Effect of 2,3-BPG on Hemoglobin Oxygen Affinity • 2,3-bisphosphoglycerate (2,3-BPG) binds allosterically with greater affinity to deoxygenated Hb than oxygenated Hb – aka. Allosteric effector • • • Interacts with β-subunits Decreases Hb O2 affinity Promotes unloading of O2 at tissues • HbF does not recognize 2,3-BPG – Allows for HbF to more readily bind O2 from the mother for fetal use Effect of 2,3-BPG on Hemoglobin Oxygen Affinity • Whole blood consists of Hb in the presence of both CO2 and 2,3-BPG • In the absence of 2,3-BPG, oxygen binding to Hb follows a rectangular hyperbola • The sigmoid binding curve is only observed in the presence of 2,3-BPG Summary • • • • • Hemoglobin has a sigmoid O2 binding curve Binding of O2 to Hb can be altered by different factors Hb can be altered by genetics (e.g. sickle cell) Oxygen is transported bound and unbound in the body and is dependent on partial pressures Total oxygen in the body is a sum of bound and unbound