* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Hematemesis, A Rare Sign of Aortic Infection
Survey
Document related concepts
Transcript
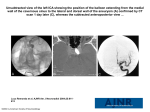
Aortic infection 111 Hematemesis, A Rare Sign of Aortic Infection Wei-Kee Huang1, Fu-Chean Chen2, Tzu-Hsuan Weng3 Ming-Hai Du1, Carlos Lam1 Non-typhoid salmonella infection of a mycotic aortic aneurysm is rare, but it is fatal when diagnosis is delayed. The clinical presentation is often nonspecific and varies widely, with fever being the most common initial presentation. Early diagnosis requires a high level of alertness and a detailed history. We report a 77 year-old man who visited our emergency room with complaints of vomiting with bloody content for two days, with increasing severity. The chest radiographs showed infiltration of the paraaortic arch left lung fields. A ruptured aneurysm was diagnosed only after contrast-enhanced computed tomography (CT) was performed. The patient received immediate surgical treatment and adequate preoperative and postoperative antimicrobial therapy. Key words: hematemesis, mycotic aortic aneurysm, non-typhoid salmonella infection Introduction Aortic infection is relatively rare but highly fatal, especially when there is formation or infection of an aneurysm (1,5). The mortality rate ranges from 20% to 53%, depending on the severity of infection, the location of the infected aneurysm, and the development of rupture, as well as whether the infection is caused by salmonella species or staphylococcus aureus (1,3,5-8). The most common pathogen is Salmonella supp. (57%), followed by Staphylococcus aureus (14%) (3) . Therefore, prompt and accurate diagnosis followed by surgical treatment and prolonged antibiotic therapy are necessary. Case Report A 77 year-old man with a history of gouty arthritis and peptic ulcer disease visited our emergency department (ED) because of persistent fever for 2 days. He had been treated by a local medical doctor under the impression of urinary tract infection, but his symptoms continued. He presented with an elevated body temperature of 38.3℃ and a stable hemodynamic status with pulse rate 92/min., respiratory rate 16/min., and blood pressure 113/53 mmHg. Routine laboratory studies showed a decreased hemoglobin level (9.6 g/dl), but a normal white blood cell count (6,630/μL). The urinanalysis was negative for infection. Hydration and antibiotic treatment with one dose of intramuscular gentamycin 80 mg were given in the ED and the fever subsided. The patient was discharged to follow-up in the outpatient department with sulfamethoxazole/trimethoprim 800 mg twice per day and acetaminophen 500 mg 4 times per day prescribed for 2 days. However, the Received: April 27, 2009 Accepted for publication: September 11, 2009 From the 1Department of Emergency Medicine, 2Department of Cardiovascular Surgery Taipei Medical University-Municpal Wanfang Hospital, Taipei 3 Department of Emergency Medicine, Taipei Medical University Hospital Address reprint requests and correspondence: Dr. Carlos Lam Department of Emergency Medicine, Taipei Medical University-Municpal Wanfang Hospital, Taipei 111 Section 3, Hsinglong Road, Taipei 116, Taiwan (R.O.C.) Tel: (02)29307930 ext 1211 Fax: (02)29301255 E-mail: [email protected] 112 J Emerg Crit Care Med. Vol. 21, No. 2, 2010 patient returned three days later with complaints of vomiting blood for two days. The patient was the adventitia of the aorta. Atheromatous plaques composed of necrotic debris and cholesterol clefts 37.5℃, pulse rate 100/min. and respiratory rate 17/min. However, the fever recurred ,with the body species. A blood culture performed in the ED during his first visit grew no bacteria. However, ambulatory, and in stable hemodynamic condition, with blood pressure 128/79 mmHg, temperature temperature elevated to 37.8℃ two hours later when acetaminophen was discontinued in the ED. Laboratory data showed a decreased hemoglobin level (9.4 g/dl), elevated blood urea nitrogen (23 mg/dl), creatinine (1.9 mg/dl), and aspartate aminotranferase (50 U/L) levels, and a normal white cell count (6,610/μL) and urinanalysis. The patient was admitted under a tentative diagnosis of upper gastrointestinal bleeding. Endoscopic examination performed on the second day showed the esophagus was not the bleeding source and had no other significant abnormality. However, a large amount of blood was noted in the stomach and a small amount in the duodenum without evidence of an active bleeding source. Therefore, a review of the disease course and previous examinations was done. A review of the chest radiographs (Fig. 1) showed blurring of the para-aortic knob lung fields, and contrast-enhanced computed tomography (Fig. 2) disclosed a wide-based 2.1 × 1.8 cm saccular aneurysm of the aortic arch just adjacent and distal to the left subclavian artery, with increased infiltrates at the left upper lobe near the aortic aneurysm, which might have been caused by previous hemorrhage into the lung parenchyma. The patient may have vomited blood after swallowing blood coughed up from the lungs. The patient had immediate surgery under a diagnosis of ruptured mycotic aneurysm of the aortic arch. The patient received a thorough debridement and resection of the infected segment of the aortic arch followed by a 24 mm Hemashield graft repair. Microscopically, the tissue specimens disclosed a picture of a ruptured atherosclerotic aneurysm of the aorta with fresh hemorrhage and acute and chronic inflammatory cell infiltraiton in were noted in the intima. Tissue culture was positive for salmonella one of the two blood cultures obtained immediately after admission to the hospital was positive for Staphylococcus epidermidis (MRSE), but this might have been caused by contamination because the rest of the blood cultures during his stay in hospital were all negative for bacterial growth. The patient received intravenous ceftriaxone 1 g q12h and vancomycin 500 mg q8h for 25 days, before and after operative treatment until skin rashes and impaired hepatic function were noted. The antibiotic treatment continued with oral ampicillin 1000 mg for 8 months. Laboratory markers for inflammation including the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) were used for monitoring. The postoperative condition was stable and the patient had an uneventful follow-up in the departments of cardiovascular surgery and infectious disease. Discussion Mycotic infection occurs in 12% of all aneurysms(4,7), and 0.8%-3.3% of patients who have reconstructive surgery for aortic aneurysm (4,8-10). Infection occurs in all portions of the aorta, most frequently in the abdominal aorta, followed by the thoraco-abdominal aorta and the thoracic aorta. Nearly all of these infections result in formation of an aneurysm, or infection of a previously existing aneurysm(1,4,5,7,9). In most patients, mycotic aneurysms are solitary lesions, but multiple aneurysms have been described (4,11). Salmonellainfected aneurysm in the thoracic aorta is rare and the prognosis is poor because the anatomical position results in a difficult surgical approach(3). Aortic infection 113 (a) (b) Fig. 1 (a) Chest radiographs (a) during the 1st and (b) 2nd ED visits. Both show a prominent aortic knob with blurring of the para-aortic lung fields (arrows) (b) Fig. 2 CT angiography (a) with sagittal reconstruction (b) shows a ruptured aneurysm at the aortic arch just adjacent to the left subclavian artery with mural thrombus and hemorrhage (arrows) 114 J Emerg Crit Care Med. Vol. 21, No. 2, 2010 The majority of salmonella infections occur in the abdominal aorta(1). The reported incidence of include fever, unremitting sepsis, and relapsing bacteraemia. Localized symptoms may be related Reported risk factors for aortic infection include diagnostic or therapeutic arterial rupture (2,15) . Rare presentations include psoas and pelvic abscesses, vertebral osteomyelitis, these infections ranges from 9 to 10% in western countries to 16.2% in a recent Taiwanese series(2). catheterization, intravenous drug use, and immunocompromised status due to chronic or neoplastic diseases such as diabetes mellitus (DM), liver cirrhosis, cancer ,or acquired immunodeficiency syndrome(1,6). DM is reported to be the most common underlying disease in salmonella aortitis. Oskoui et al. (5) reported that 28% of 98 patients with salmonella aortitis had DM. Bacterial seeding of the aortic wall can occur by hematogenous spread to the intima or vasa vasarum such as in pneumonia or systemic sepsis, by lymphatic spread such as in tuberculous aortitis (4,12) , or by direct extension from an adjacent focus such as in purulent pericarditis or osteomyelitis (4,12,13) . The thin endothelial intimal lining of the aorta is generally highly resistant to infection (4,14), but disruption of this barrier by atherosclerosis reduces this resistance. The normal aortic intima can also be subject to bacterial invasion causing degenerative changes in the arterial wall which result in aneurysm formation and rupture(4,12,14). The latter may occur as early as 1 week after the onset of aortitis, and may be heralded by pericardial or pleural effusion (4,12) . Infection of a pre-existing aortic aneurysm is reported to occur in 3% of patients with aneurysm (1,6) . Salmonella species have a predilection for diseased arteries and are associated with a high incidence of early aneurysm rupture(4,12). Hence, salmonella sepsis should alert the physician to the possibility of salmonella aortitis, particularly i n t h e e l d e r l y, i n w h o m t h e p r e v a l e n c e o f atherosclerosis is increased. Manifestations of endovascular infections can be protean and non-specific. Common presentations to pressure effects from aneurysms, contagious extension of infection, or impending aneurysmal endobronchial masses(2), hemoptysis(2), and gastrointestinal bleeding due to aortobronchial or aortoduodenal fistula formation(2,15). The presence of blood in our patient’s stomach and duodenum were not due to bleeding from the fistula tract but from swallowing blood after hemoptysis. Failure to consider these unusual complications of non-typhoid salmonella bacteraemia can lead to catastrophic consequences. Routine chest radiographs can be significant if they show prominent periaortic lung tissue infiltration. Computed tomography is usually the initial radiographic study of choice for detection of mycotic aneurysms. Transesophageal echocardiography is the diagnostic method of choice for ruling out endocarditis(4,14) and a negative result should prompt the operator to examine the thoracic aorta for evidence of a mycotic aneurysm. Aortography may be useful for excluding multiple mycotic aneurysms, and outlining the relation of the mycotic aneurysm to the major branches of the aorta (4,8-11,13). Endoscopic study of the upper gastrointestinal system may be necessary if there is any doubt in differentiating hemoptysis from hematemesis. Therefore, a mycotic aneurysm may not be suspected initially, as in out patient, but the diagnosis is made eventually while searching for the source of persistent fever and hematemesis. A high degree of awareness remains an indispensable prerequisite for early diagnosis. Laboratory investigation may reveal nonspecific findings such as leukocytosis, or an increased ESR. Negative blood cultures have been reported in up to 25% of cases and may be caused by antibiotic pretreatment and anaerobic organisms. This may also could Aortic infection 115 account for negative culture results in surgically removed pathological tissue sections(4,8,9). or reduced immunity, a detail medical history is, therefore, necessary. The appearance of periaortic admission(4,10), and appropriate antibiotic therapy. Complete excision of the affected aorta is the key a targeted CT scan should be done urgently. Immediate surgical treatment must be available Current management of mycotic aneurysm c o n s i s t s o f s u rg e r y a s e a r l y a s t h e d a y o f to curative treatment. It is the definitive treatment even when the infection appears to be controlled with antibiotics, because adequate infection control does not prevent aneurysm rupture(4,18). There is no consensus on the duration of antibiotic therapy(4,10). The length of preoperative antibiotic usage can range from 24 hours to 30 days (4,10,13), whereas postoperative treatment can range from 6 weeks to 12 months. When the source of infection is not known, life-long prophylactic antibiotic therapy is recommended, particularly if disease is caused by salmonella species, and if there is active infection (regardless of cause) at the time of surgery (4,8,9). In postoperative follow-up serial non-invasive imaging studies should be done to exclude recurrent infection and laboratory markers of inflammation should be monitored(4,8). Recurrent or persistent infection is the leading cause of death after successful surgery. Conclusion We reported a rare case of non-typhoid salmonella mycotic aneurysm of the aortic arch in a patient presenting with recurrent fever despite antipyretic treatment, and an initial presentation of hematemesis. Non-typhoid salmonella bacteraemia is frequently associated with fever or sepsis, unexplained symptoms of chest, abdominal or back pain, hemoptysis, and rarely, hematemesis. Whether or not blood is coughed up or vomited, an alert physician should perform a prompt and thorough evaluation for possible aortic infection in the ED, especially in patients over 50 years old, and in those with risk factors for atherosclerosis infiltrates on routine chest radiography should arouse the suspicion of the ED physician and with adequate preoperative and postoperative antimicrobial therapy. References 1. Kimura N, Yamaguchi A, Noguchi K, Adachi K, Adachi H, Ino T. Type B aortic dissection associated with Salmonella infection. Gen Thorac Cardiovasc Surg 2007;55:212-6. 2.Wong Stella PY, Lai Thomas KK, Ng WL, Luk WK. Non-typhoid Salmonella mycotic aneurysm of the aortic arch. Hong Kong Med J 2007;13:234-7. 3.Hsu RB, Lin FY. Infected aneurysm of the thoracic aorta. J Vasc Surg 2008;47:270-6. 4.Malouf JF, Chandrasekaran K, Orszulak TA. Mycotic aneurysms of the thoracic aorta: a disgnosti challenge. Am J Med 2003;115:489-96. 5.Oskoui R, Davis WA, Gomes MN. Salmonella aortitis. A report of a successfully treated case with a comprehensive review of the literature. Arch Intern Med 1993;153:517-25. 6.Gomes MN, Choyke PL, Wallace RB. Infected aortic aneurysm, A changing entity. Ann Surg 1992;215:435-42. 7.Parkhurst GF, Decker JP. Bacterial aortitis and mycotic aneurysm of the aorta. Am J Pathol 1955;31:821-35. 8.Muller BT, Wegener OR, Grabitz K, et al. Mycotic aneurysms of the thoracic and abdominal aorta and iliac arteries: experience with anatomic and extra-anatomic repair in 33 cases. J Vasc Surg 2001;33:106-13. 9.Gross C, Harringer W, Mair R et al. Mycotic 116 J Emerg Crit Care Med. Vol. 21, No. 2, 2010 a n e u r y s m s o f t h e t h o r a c i c a o r t a. E u r J Cardiothorac Surg 1994;8:135-8. 10.Chan FY, Crawford ES, Coselli JS, et al. In situ prosthetic graft replacement for mycotic aneurysm of the aorta. Ann Thorac Surg 1989;47:193-203. 11. Chen YF, Lin PY, Yen HW, Lin CC. Double mycotic aneurysms of the ascending aorta. Ann Thorac Surg 1997;63:529-31. 12.Gomes MN, Choyke PL, Wallace RB. Infected aortic aneurysm: CT doagnosis. J Cardiov Surg (Torino) 1992;33:684-9. 13.Pasic M, Carrel T, von Segesser, Turina M. In situ repair of mycotic aneurysm of the ascending aorta. J Thorac Cardiov Surg 1993;105:321-6. 14. Vilacosta I, Bustos D, Ciguenza R, et al. Primary mycotic aneurysm of the ascending aorta diagnosed by transesophageal echocardiography. J Am Soc Echocardiogr 1998;11:216-8. 15.Fernandez Guerrero ML, Aguado JM, Arribas A, Lumbreras C, de Gorgolas. The spectrum of cardiovascular infections due to salmonella enterica: a review of clinical features and f a c t o r s d e t e r m i n i n g o u t c o m e. M e d i c i n e 2004;83:123-38. 16.S h r a g e r J B, Wa i n J C, W r i g h t C D, e t a l. O m e n t u m i s h i g h l y e ff e c t i v e i n t h e management of complex cardiothoracic surgical problems. J Thorac Cardio Surg 2003;125:526-32. 17.K o t z a m p a s s a k i s N, D e l a n a y e P,M a s y F, Creemers E. Endovascular stent-graft for thoracic aorta aneurysm caused by salmonella. Eur J Cardio Surg 2004;26:255-7. 18.Knosalla C, Weng Y, Yankah AC, et al. Using aortic allograft material to treat mycotic aneurysms of the thoracic aorta. Ann Thorac Surg 1996;61:1146-52. 主動脈感染 117 吐血、罕見之主動脈細菌感染徵象的個案報告 范為其1 陳復銓2 翁子軒3 杜明海1 林樹基1 主動脈非典型沙門氏桿菌感染是一種罕見但卻可致命的疾病,尤其因未能早期診斷因而延誤手術治 療。此疾病的臨床病徵經常是非特定性且多變的,一般常見症狀為發燒,直到較特定性病徵如胸痛的出 現,這通常表示動脈瘤擴大或破裂,而急診醫療人員需具備高度警覺性,才能在此發病初期診斷此疾 病。本文報告一位77歲男性患者,因發燒,吐血兩天且日趨嚴重,到本院急診就診。胸部X光檢查顯示 主動脈弓旁之左側肺部浸潤,雖然經由造影加強之電腦斷層檢查後確認診斷。 關鍵詞: 吐血,主動脈瘤,非典型沙門氏桿菌感染 收件:98年4月27日 接受刊載:98年9月11日 台北醫學大學市立萬芳醫院 1急診醫學部 2心臟血管外科部 3台北醫學大學附設醫院急診醫學部 通訊及抽印本索取:林樹基醫師 116台北市文山區興隆路三段111號 台北醫學大學市立萬芳醫院急診醫學部 電話:(02)29307930轉1211 傳真:(02)29301255 E-mail: [email protected]