* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Treatment Options for Tooth Discoloration and Remineralization
Special needs dentistry wikipedia , lookup
Dental degree wikipedia , lookup
Focal infection theory wikipedia , lookup
Water fluoridation in the United States wikipedia , lookup
Water fluoridation wikipedia , lookup
Fluoride therapy wikipedia , lookup
Crown (dentistry) wikipedia , lookup
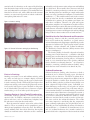
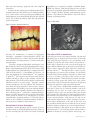
Earn 4 CE credits This course was written for dentists, dental hygienists, and assistants. Treatment Options for Tooth Discoloration and Remineralization A Peer-Reviewed Publication Written by Dr. Fiona M. Collins, MBA, MA PennWell is an ADA CERP recognized provider ADA CERP is a service of the American Dental Association to assist dental professionals in identifying quality providers of continuing dental education. ADA CERP does not approve or endorse individual courses or instructors, nor does it imply acceptance of credit hours by boards of dentistry. PennWell is an ADA CERP Recognized Provider Concerns of complaints about a CE provider may be directed to the provider or to ADA CERP at www.ada.org/goto/cerp. PennWell is an ADA CERP Recognized Provider 1-888-INEEDCE Go Green, Go Online to take your course This course has been made possible through an unrestricted educational grant. The cost of this CE course is $59.00 for 4 CE credits. Cancellation/Refund Policy: Any participant who is not 100% satisfied with this course can request a full refund by contacting PennWell in writing. Educational Objectives Overall goal: The purpose of this article is to provide dental professionals with information on the etiology and treatment of tooth discoloration and loss of tooth structure. Upon completion of this course, the clinician will be able to do the following: 1. List the etiologies for tooth discoloration. 2. List traditional therapies available for the treatment of tooth discoloration and describe calcium and phosphate technology and the clinical application of this technology in treating and preventing discoloration of teeth. 3. List the etiologies for tooth demineralization. 4. List traditional and new therapies available for the prevention of demineralization and for remineralization, and describe how these work. Abstract Fully developed teeth have a finite quantity of enamel and cementum, and internally consist of dentin and pulp. Over time, patients may experience loss of tooth structure due to caries, erosion, abrasion or attrition. Patients also experience tooth discoloration due to aging changes as well as extrinsic and intrinsic staining. The prevention and treatment of these conditions is clinically and esthetically desirable. Traditional treatment and prevention options have included fluoride and chemotherapeutics to help prevent tooth structure loss, and abrasives and hydrogen peroxide to treat tooth discoloration and staining. Recently introduced calcium and phosphate technologies have increased the treatment options available. Introduction Fully developed newly-erupted teeth have a finite quantity and depth of enamel and cementum that overlie dentin, and internally have large pulp chambers. The enamel structure comprises approximately 97% hydroxyapatite crystals containing calcium and phosphate minerals (as well as traces of other minerals and organic material). Dentin and cementum contain a higher proportion of organic material compared to inorganic material (mineral content). Over a period of many years, the pulp chamber contracts while secondary dentin is simultaneously laid down internally in areas that were originally occupied by the outer region of the pulpal tissue.1 Dental disease and conditions resulting in the loss of tooth structure posteruption include caries, erosion, abrasion and attrition. Aging Changes The surface morphology of teeth influences both the perception of color and the amount of extrinsic stain that accumulates – smooth surfaces with few or no crevices or cracks are less likely to develop extrinsic stain (or, via cracks, intrinsic stain) than are rough surfaces. In newly erupted 2 teeth normal enamel reflects and refracts light, resulting in a lustrous and glossy appearance.2 As enamel ages, it loses its initial surface texture. This causes a reduction in light refraction and reflection and results in the light penetrating deeper into the tooth. As a consequence, the tooth appears less lustrous and shade differences become more obvious, leading to the appearance of a darker tooth. In addition, the translucent incisal edge increases in area.3,4 Tooth Discoloration and Staining Changes in the coloration of teeth occur due to extrinsic and intrinsic staining in addition to the aging changes described above. Table 1. Factors in Tooth Discoloration and Staining Intrinsic Extrinsic Aging Aging changes of tooth Trauma Surface changes (defects) Specific medications Red wine Thickness of hard tissue Tobacco Structure of hard tissue Coffee and tea Enamel cracks Foods rich in polyphones Specific medications Chlorhexidine gluconate rinse Metabolic diseases Level of oral hygiene Systemic conditions Intrinsic Staining Intrinsic staining starts from inside the tooth or results from stain penetrating into the dentin from the surface of the tooth. The use of certain medications such as tetracycline during tooth development also causes intrinsic staining. Genetics plays a role, and some metabolic disease and systemic conditions result in tooth discoloration during development.5 Finally, trauma to teeth can result in nonvitality and a gradual graying and blackening of the crown of the tooth. Treating Intrinsic Tooth Discoloration While extrinsic discoloration and staining has the potential to be treated chemically and/or mechanically, mechanical treatment will not treat intrinsic staining. Intrinsic tooth discoloration can be treated using carbamide peroxide (which breaks down to hydrogen peroxide and urea) or hydrogen peroxide compounds in either an in-office or at-home procedure. In-office treatments will typically use a higher concentration of whitening agent, usually hydrogen peroxide in concentrations of up to 35%. Irrespective of the selected method, the mechanism of action is the same – hydrogen peroxide degrades into free radicals that break double bonds in the stain, thereby lightening and removing the color from the stained material. In the case of www.ineedce.com nonvital teeth, discoloration can be removed by bleaching from the internal aspect of the crown of the tooth (provided root canal obturation was adequate). This method has been found to be effective and, where sufficient tooth structure remains, can conservatively provide an esthetic result without requiring fabrication of a crown.6 Figure 1a. Intrinsic staining in dentifrices to help remove stain and prevent stain buildup on a daily basis. Recent studies have shown not only that dentifrices containing no abrasive result in stain accumulation, but that patients are unwilling to comply with regular home care using a nonabrasive toothpaste even under study conditions.10,11,12 This would result not only in the accumulation of stain, but also the accumulation and maturation of biofilm since patients do not perform good home care under these conditions. Therefore, for good oral hygiene and patient compliance it is important to be able to achieve cleaning and stain prevention and removal, while preserving tooth structure. A dentifrice should ideally contain sufficient cleaning power to remove and help prevent staining without being so abrasive that it results in loss of tooth structure.13,14 Dentifrice Use for Stain Removal and Prevention Image courtesy of Howard E. Strassler, DMD Figure 1b. Removal of intrinsic staining by toothwhitening Image courtesy of Howard E. Strassler, DMD Increasingly, abrasives with fine, rounded particles have been used in modern-day dentifrices, to gently remove stain and simultaneously help impart a polish that gives the appearance of whiter teeth. Typical agents include calcium phosphate, calcium carbonate and sodium bicarbonate. The Radioactive Dentin Abrasion (RDA) measures how abrasive a toothpaste is. The cleaning ability of a dentifrice depends on both its cleaning power and its abrasivity – a dentifrice with high cleaning power and low abrasivity will minimize the risk of abrasion while maximizing cleaning efficacy. A European study in 1997 found that most of the cleaning obtained from a dentifrice was through abrasion.15 An exception is dentifrice containing sodium bicarbonate (baking soda), which has been used in dentifrices for almost 30 years. Sodium Bicarbonate Extrinsic Staining Smoking can result in severe and stubborn staining, while tea, coffee and red wine are common culprits associated with surface staining.7 Eating specific types of food results in surface staining of teeth. Good oral hygiene helps reduce and prevent staining, and conversely the propensity to stain is increased if oral hygiene is poor. Medium- to long-term use of chlorhexidine gluconate rinse is known to result in tooth staining, as is use of other specific therapeutics.8 Treating Extrinsic Stain/Tooth Discoloration Chemically, hydrogen peroxide is effective for treating extrinsic stain.9 In addition, surfactants contained in dentifrices and rinses, such as sodium lauryl sulphate, help lift and remove stain from the tooth surface. Physical removal of extrinsic stain can be achieved with abrasives. These are routinely used in prophylactic pastes during dental prophylaxis where they have the ability to remove stubborn stain due to their abrasivity, that regular dentifrices would not remove. Abrasives are also contained www.ineedce.com Sodium bicarbonate (baking soda) functions as both a mechanical and a chemical cleaning agent. Mechanical cleaning relies on abrasives. The RDA of sodium bicarbonate ranges from 30-40, compared to an RDA of 70-110 in typical dentifrices. Studies show that while it has low abrasivity, baking soda has excellent cleaning ability even when compared to higher RDA agents. Controlled-condition laboratory studies using several grades of sodium bicarbonate differing in particle size, with water and glycerin to simulate dentifrice and tooth brushing, determined that the mean ratio of abrasion to cleaning power was 10.2, compared to 1.7 for calcium pyrophosphate (which is used as a reference material for abrasiveness studies).16 Little difference was found in the results across the various particle sizes used for the sodium bicarbonate. The results were more impressive considering that the comparative agent, sodium pyrophosphate, has a higher RDA and, additionally, prevents stain buildup by means of chelation as well as abrasion.17,18,19 Zambon et al. found that use of 52% and 65% sodium bicarbonate for 6 months resulted in a positive shift in the composition of the intraoral microbial 3 flora and reduced plaque, gingivitis and stain compared to baseline.20 It is believed that cleaning power rather than abrasivity is an important factor in the effectiveness of sodium bicarbonate as a stain remover and in preventing stain buildup, and that bicarbonate cleans through both physical and chemical action. The sodium bicarbonate helps lift stain from the surface of the tooth. Foundation. It is contained in dentifrice (ARM & HAMMER® Age Defying, Church & Dwight) as well as in-office preventives and prophy pastes (Enamel ProTM and Enamel ProTM Varnish, Premier Dental), tooth-whitening agents (Zoom® 2 Day White and Nite White® with ACP, Discus Dental), and other products. Figure 3. SEM of ACP Figure 2. Extrinsic staining and abrasion Image courtesy of Salim Nathoo, DDS, PhD Recently, the introduction of calcium and phosphate technology - amorphous calcium phosphate (ACP) - has offered the potential for esthetic and preventive benefits from dentifrices and other products, as well as from traditional therapies. Two other calcium and phosphate technologies available include Novamin® and casein phosphopeptide –amorphous calcium phosphate (CPP-ACP). The casein in CPP is obtained from cow’s milk and forms a complex with ACP. CPP-ACP (Recaldent®) is bioavailable and releases calcium and phosphate for remineralization.21 It is contained in MI PasteTM (GC America) and chewing gum (Trident, Cadbury Adams) as well as in glass ionomer cements and other products. CPP-ACP has been shown to remineralize subsurface lesions in in vitro testing.22 Novamin contains calcium, phosphorus, silica and sodium. The Novamin releases these ions upon exposure to saliva or water, aiding in remineralization and helping to prevent demineralization. Studies of Novamin-containing dentifrice have found it to have a whitening effect.23 A Novamin-containing dentifrice that also includes fluoride has been found to remineralize surface lesions in vitro by 41.9% compared to 24.9% for a control fluoride-containing dentifrice.24 CPP-ACP and Novamin are not available in sodium bicarbonate dentifrices; they are however available in other dentifrices. Amorphous Calcium Phosphate Amorphous calcium phosphate (ACP) consists of an unstructured form of calcium phosphate molecules (Figure 3) and was developed by the American Dental Association 4 Image courtesy of the American Dental Association Foundation The role of ACP in dentifrices ACP offers preventive and cosmetic benefits. Calcium and phosphate ions are deposited onto the surface of the tooth with ACP-delivering dentifrice use, precipitating ACP globules. If scratches and surface defects are present on a tooth, light is dispersed in such a way that the tooth can appear dull and darker than it otherwise would. Filling in surface defects changes the light dispersion, thereby improving esthetics.25,26 The light becomes scattered, giving the appearance of a surface gloss and luster. In situ testing has found that ACP precipitates and lodges in defects such as crevices and pits; it is believed this converts to mineral hydroxyapatite.27 While hydrogen peroxide and other chemical cleaning agents will remove stain, they cannot repair surface defects that alter the dispersion of light and therefore the appearance of the (nonstained) tooth. One single-center, double-blind randomized trial assessed twice-daily use of a fluoride dentifrice with ACP technology for its effect on surface gloss and surface roughness. Surface gloss was measured using the enamel gloss standard test. The subjective improvement using the test dentifrice was found to be 73.8% compared to 17.6% for the control dentifrice, with between 76% and 83% of teeth showing improvement. Surface roughness was measured using a profilometer at the start of the study and one and three months after use of either the test or control dentifrice. Surface roughness was reduced significantly using the test dentifrice, but did not decrease with use of the control dentifrice.28 ACP will precipitate onto the tooth as globules as shown by scanning electron microscopy. Imwww.ineedce.com provements in luster and deposition of ACP into surface defects have also been found to occur using prophy paste delivering ACP.29 Figure 4. ACP precipitation onto dentin in in vitro study Image courtesy of American Dental Association Foundation Intraorally, ACP has the fastest rate of formation and dissolution of the calcium phosphate compounds.30 As pH increases, ACP precipitates onto the tooth surface. The inclusion of carbonate results in a more rapid increase in pH. Carbonates have been shown to control the precipitation of ACP onto tooth surfaces.31,32 Thus, the combination of bicarbonate and ACP delivery in dentifrice has been found to help the presence of ACP at the site and the supersaturation of calcium and phosphate ions. of children between the ages of 2 and 5 have experienced decay.33 By age 19, 68% of adolescents have experienced caries in the permanent dentition.34 The patient’s level of plaque control (bacterial load), salivary flow and composition, diet (amount and frequency of intake of fermentable carbohydrates), tooth structure, and use of home care products and therapeutics determine whether demineralization occurs. These factors also determine whether this process progresses to subsurface demineralization and, eventually if unhindered, frank cavitation. Hydroxyapatite crystals are stable at a pH of 7 (neutral). When acid attacks occur, dissolution of the hydroxyapatite crystals starts when the pH has decreased to around 5.5. Demineralization results in the loss of calcium, phosphate and hydroxyl ions from the hydroxyapatite crystals. Following this acid attack, the pH rebounds. Normalization of the pH takes longer if salivary flow is reduced, increasing the time during which demineralization can occur. Additionally, a reduced salivary flow results in fewer minerals being available from saliva that would aid remineralization – namely calcium and phosphate. Natural saliva is supersaturated with calcium and phosphate that over time help repair defects, including in the absence of fluoride.35 Salivary flow is an important determinant of remineralization, and this helps explain the rampant caries seen in xerostomic patients.36 An increased concentration of calcium and phosphate at the tooth surface would strengthen the repair of the enamel surface. Figure 6. Dynamics of demineralization and remineralization Figure 5. Bicarbonate, ACP and dentifrice activity Loss of Tooth Structure Loss of tooth structure over time can occur as a result of the caries process, abrasion, erosion or attrition. Dental caries is a chronic disease experienced by the majority of the population. Most children are infected with cariogenic bacteria before the age of 2, and around 28% www.ineedce.com Intact enamel is the hardest substance in the body. Dentin is considerably softer; the softest and thinnest layer of dental hard tissue is the cementum. Studies have found that enamel is approximately 4 to 10 times harder than dentin, depending on the locations of the samples of the enamel and the dentin within the tooth.37 As enamel is lost and dentin exposed, there results increased susceptibility to loss of dental hard tissue. The presence of erosion complicates the clinical picture. Tooth erosion occurs when acids of nonbacterial origin (i.e., not produced by cariogenic bacteria)38 demineralize 5 the exposed surface of the tooth. These acids may be the result of regurgitation of stomach acid such as occurs due to gastro-esophageal reflux disease (GERD) or due to the frequent vomiting associated with bulimia.39,40 External sources of acids causing erosion of dental hard tissues include soda pop, which currently accounts for around 28% of all beverages consumed, as well as other dietary habits and environmental hazards.41,42,43 Erosion results in surface loss, removes surface minerals and results in the tooth losing its luster and glossy appearance;44 it also softens mineralized tissue, increasing susceptibility to (further) demineralization, abrasion and attrition.45,46 Abrasion physically wears away the surface layer of the tooth and in the long term results in the intraoral exposure of the softer dentin. Attrition also results over time in the exposure of dentin, and can occur due to bruxism or mastication of rough high-fiber foods over the long term. As abrasion proceeds, the perception of tooth color changes – abrasion results in less enamel being present and dentin shining through the thinner layer of enamel or being exposed, or only dentin being present. Fluoride The first use of fluoride as a caries preventive occurred more than a hundred years ago in Europe. Fluoridens was a fluoride-containing powder used in the late 1800s. During the 1950s and 1960s, acidulated phosphate fluoride, neutral sodium fluoride, amine fluoride and stannous fluoride were investigated and introduced as in-office topical agents and home-use products. The frequency of in-office fluoride treatments differs with each patient’s level of risk (typically from zero up to four times per year). Figure 7. Timeline of preventive measures Late 1800s Fluoridens 1950s and 60s Topical fluorides 1940s Water fluoridation 1990s, 2000s Calcium and phosphate technologies Table 2. Tooth structure loss Caries Surface and subsurface loss Abrasion Surface loss Attrition Surface loss Erosion Surface loss; contributes to other causes Remineralization and the Prevention of Demineralizaton Demineralization can be counteracted and reversed through remineralization at the time of acid attacks as well as in the early stages of hard tissue demineralization. Conditions that discourage demineralization and favor remineralization include a reduced load of cariogenic bacteria, good salivary flow, the presence of calcium, phosphate and fluoride, as well as the use of chemotherapeutic agents. Antibacterial agents have been used to prevent demineralization. Chlorhexidine gluconate rinse and povidone iodine reduce the load of cariogenic (and periodontopathic) bacteria present, and have been found to help prevent caries in high-risk children and in high-risk adults with severe xerostomia.47,48 Xylitol occurs naturally and is known to be antimicrobial and a caries preventive. A three-year study of a dentifrice containing 10% xylitol and fluoride found a 12% reduction in decayed and filled surfaces after accounting for fluoride in the control dentifrice.49 Xylitol is used in chewing gums and mints, and as a sweetener in a number of oral care products in the United States. The single greatest advancement in the last century for caries prevention was the use of fluoride. 6 Dentifrices provide patients with a regular daily supply of fluoride, and it has been stated that fluoride-containing dentifrices and rinses have contributed more to the reduction of caries in the general population than other measures.50 The investigation recently of calcium and phosphate technology has shown the ability to enhance prevention as well as esthetics. Use of fluoride-containing dentifrices that include calcium and phosphate and deliver ACP helps the supply of calcium and phosphate ions for the strengthening of enamel. A risk assessment is required to determine the appropriate preventive measures for individual patients. The Role of Calcium and Phosphate in the Demineralization-Remineralization Balance Normal saliva is supersaturated with calcium and phosphate ions that help prevent dissolution of hydroxyapatite crystals. Calcium from milk and cheese has been found to mitigate the effects of erosion.51 Using calcium and phosphate solutions, testing has shown that using these sequentially (first calcium, then phosphate) results in the rapid deposition of amorphous calcium phosphate and its conversion to hydroxyapatite.52 The use of calcium and phosphate and ACP delivery has been studied in dentifrices in vitro and in vivo. High concentrations of calcium and phosphate can exist at low pH, and as the pH increases, the calcium and phosphate precipitate. Supersaturation of calcium and phosphate adjacent to the tooth at low pH helps prevent dissolution www.ineedce.com of crystals. This is a concentration gradient effect. As the pH increases, the balance changes and ACP and fluoride are incorporated on the surface of the tooth. ACP can then convert to hydroxyapatite, which is less soluble than ACP. This can help prevent demineralization, since, at a given pH, ACP is more soluble than hydroxyapatite crystals, with the result that the ACP would dissolve first and supersaturate the area with calcium and phosphate ions, helping to prevent dissolution of the hydroxyapatite crystals. The amorphous structure of ACP also enables fluoride and other ions to be incorporated into it. In vitro testing has found that the presence of ACP can increase fluoride bioavailability and uptake in ACP-delivering products.53,54 Dentifrice containing calcium and phosphate ions has been found to increase the bioavailability of fluoride, resulting in increased uptake of fluoride in in vitro studies using enamel cores. An uptake of more than 5000 ppm compared to just over 1900 ppm for a control dentifrice was found, and the solubility of enamel was reduced.55 Table 3. Fluoride uptake and solubility of enamel Fluoride Uptake (ppm) 6000 5031 5000 4000 3000 1915 2000 1000 0 -1000 -3.1 Non-Fluoride Fluoride Liquid Calcium and Fluoride Dentifrice Type In separate in vitro studies, eroded enamel discs were exposed to a test dentifrice containing liquid calcium, phosphate and fluoride, and it was found that the test dentifrice increased hardness more than the fluoride-containing dentifrice. In addition, after pretreatment with soda pop, the test dentifrice increased hardness of the enamel discs by 12% compared to 7% for the control dentifrice, indicative of a greater remineralizing effect.56 Figure 8. In vitro surface hardness using liquid calcium-containing dentifrice www.ineedce.com Clinical Application – In-office and Home Use Demineralization and discoloration of teeth can be treated and/or prevented using suitable in-office and home-care therapies that effectively and safely prevent and treat these conditions. In the case of home care, an ideal would be for the same care to prevent demineralization, aid remineralization, prevent tooth discoloration and remove any discoloration present. One option that utilizes over-the-counter dentifrice without an additional steps being required by the patient, would be the use of products containing fluoride and delivering ACP. Based on in vitro studies, in-office use of prophylactic paste delivering ACP increases fluoride availability where prophylaxis is indicated and has also been shown to result in the filling in of surface defects and an improvement in luster, which would enhance esthetics since the light would be dispersed over a smoother surface. Home use of dentifrice delivering ACP to the tooth (ARM & HAMMER® Age Defying) has been shown in in vivo and in vitro studies to improve appearance and surface luster by this mechanism and, based on in vitro studies, also results in supersaturation of the area with calcium and phosphate. Clinically, a combined in-office and home care regimen is suitable for patients presenting with extrinsic staining, loss of surface luster and hard tissue, and patients at risk for demineralization. Depending on caries risk, increased levels of topical fluoride, frequency of in-office fluoride, and use of antimicrobials may also be indicated. Table 4. Indications for patients for in-office and home use regimen • Extrinsic staining • Loss of surface area • Loss of enamel luster • Risk of demineralization Summary Loss of tooth structure occurs as a result of dental disease and conditions that include caries, erosion, abrasion and attrition. Tooth discoloration and staining is prevalent, and varies with age and habits and by individual patient. The treatment of tooth discoloration and the prevention and treatment of caries have been central in dentistry for a number of decades. Fluoride was first routinely used more than half a century ago, and tooth whitening has become a routine procedure. Extrinsic stain can be treated chemically and mechanically. In addition to stain removal, the filling in of surface defects has been found to alter light diffraction and dispersion, altering the perception of tooth color and improving surface luster. Recent innovations have resulted in products containing calcium and phosphate technology as well as fluoride. These offer cosmetic benefits and increase the availability of calcium, phosphate and fluoride for the strengthening of enamel. 7 References 1 Kvaal SI, Kolltveit KM, Thomsen IO, Solheim T. Age estimation of adults from dental radiographs. Forensic Sci Int. 1995;74(3):175-85. 2 Ten Bosch JJ, Coops CC. Tooth color and reflectance as related to light scattering and enamel hardness. J Dent Res. 1995;74:374-80. 3 Shimada K, Kakehashi Y, Matsumura H, et al. In vivo quantitative evaluation of tooth color with hand-held colorimeter and custom template. J Prosthet Dent. 2004;1991:389-91. 4 Nathoo SA, Chmielewski MB, Rustogi KN. Clinical evaluation of Colgate Platinum Professional Toothwhitening 5 6 7 8 System and Rembrandt Lighten Bleaching Gel. Compend Contin Educ Dent. 1994;15(S17):S640-45. Pindborg JJ. Pathology of the dental hard tissues. Copenhagen:Munksgaard, 1970. p221. Amato M, Scaravilli MS, Farella M, Riccitiello F. Bleaching teeth treated endodontically: long-term evaluation of a case series. J Endod. 2006;32(4):376-8. Nathoo SA. The chemistry and mechanisms of extrinsic and intrinsic discoloration. J Am Dent Assoc. 1997;128:6S–10S. McCoy LC, Wehler CJ, Rich SE, Garcia RI, Miller DR, Jones JA. Adverse events associated with chlorhexidine use: results from the Department of Veterans Affairs Dental Diabetes Study. J Am Dent Assoc. 2008;139(2):178-83. 9 Joiner A, Thakker G. In vitro evaluation of a novel 6% hydrogen peroxide tooth whitening product. J Dent. 2004;32 Suppl 1:19-25. 10 Hefferren J, and Dentifrice Function Committee. Clinical studies to determine clinical response to nonabrasive dentifrice use. J Dent Res. 1996;75:45. 11 Cooley WE, Sturzenberger P, Hefferren J. and Dentifrice Function Committee. Clinical response of non-abrasive dentifrice use in adults. J Dent Res. 1996;75:87. 12 Lobene R, Hefferren J, and Dentifrice Function Committee. Clinical response of non-abrasive dentifrice use in dental hygienists. J Dent Res.1996;75:88. 13 Wülknitz P. Cleaning power and abrasivity of European toothpastes. Adv. Dent Res. 1997;11:576-579. 14 Miller WD. Experiments and observations on the wasting of tooth tissue variously described as erosion, abrasion, chemical abrasion, denudation, etc. Dent Cosmos. 1907;49:1–23, 109–124, 225–247. 15 Wülknitz P. Cleaning power and abrasivity of European toothpastes. Adv. Dent Res 1997;11:576-579. 16 International Standards Organization ISO 11609, 1995 Dentistry—toothpaste—requirements, test methods and marking. 17 Berg ML, Ersen E, Smith J, Coffman L, Hefferren J. Clinical functionality of seven grades of sodium bicarbonate in the same toothpaste formulation. J Dent Res. 2003;82:A #386. 8 18 Smith J, Ersen E, Coffman L, Berg ML, Hefferren J. Cyclic laboratory model to measure the chemical cleaning powder of seven grades of sodium bicarbonate. J Dent Res. 2003;82:A #384. 19 Stookey GK, Burkhard TA, Schemehorn BR. In vitro removal of stain with dentifrices. J Dent Res. 1982;61:1236-9. 20 Zambon JJ, Mather ML, Gonzales Y. A microbiological and clinical study of the safety and efficacy of baking soda dentifrices. Comp Cont Ed Dent. 1997;18(21):S39-44. 21 Shen P, Cai F, Nowicki A, Vincent J, Reynolds EC. Remineralization of enamel subsurface lesions by sugarfree chewing gum containing casein phosphopeptideamorphous calcium phosphate. J Dent Res. 2001 Dec;80(12): 22 Cochrane NJ, Saranathan S, Cai F, Cross KJ, Reynolds EC. Enamel subsurface lesion remineralisation with casein phosphopeptide stabilised solutions of calcium, phosphate and fluoride. Caries Res. 2008;42(2):88-97. Epub 2008 Jan 15. 23 Clinical Evaluation for Tooth Whitening of a Bioglass® (NovaMin) Containing Dentifrice. 24 Alaudin SS, Fontana M. Evaluation of NovaMin® as an adjunct to fluoride for caries remineralization. Novqamin Research Report. Available at: http://novamin.com/pdf/ Evaluation_of_NovaMin_As_An_Adjunct_to_Fluoride. pdf. Accessed April, 2008. 25 Tung MS, Eichmiller FC. Dental applications of amorphous calcium phosphates. J Clin Dent. 1999;10(1 spec no):1-6. 26 Schemehorn BR, Orban JC, Wodd GD, et al. Remineralization by fluoride enhanced with calcium and phosphate ingredients. J Clin Dent. 1999;10(1 spec no):13-6. 27 Tung MS, Eichmiller FC. Amorphous calcium phosphates for tooth mineralization. Comp Cont Educ Dent. 2004;25(9, Suppl. 1);9-13. 28 Muñoz CA, Stephens JA, Proskin HM, Ghassemi A. Clinical efficacy evaluation of a fluoride dentifrice containing calcium, phosphate, and sodium bicarbonate on surface-enamel smoothness and gloss. Comp Cont Ed Dent. 2004;25(9)(Suppl. 1):32-43. 29 Tung MS, Eichmiller FC. Amorphous calcium phosphates for tooth mineralization. Comp Cont Educ Dent. 2004;25(9, Suppl. 1);9-13. 30 Eanes ED. Amorphous calcium phosphate thermodynamic and kinetic considerations. In: Amjad Z, ed. Calcium, phosphates in biological and industrial sciences. Boston, Mass:Kluwer academic publishers;1997:21-39. 31 Munoz CA, Stephens J, Wilson A, et al. Tooth surface restoration with a bicarbonate-dentifrice containing calcium and phosphate (Abstract). J Dent Res. 2004;83(spec iss). Abstract 2115. 32 Muñoz CA, Dahlke H, Charig A, et al. Surface mineralization by a two-phase baking soda toothpaste www.ineedce.com 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 containing calcium. J Dent Res. 2004;83(spec iss). Abstract 2117. Centers for Disease Control. http://www.cdc.gov/ OralHealth/topics/child.htm. Accessed May 2008. Ibid. Ogaard B, ten Bosch JJ. Regression of white spot enamel lesions: A new optical method for quantitative longitudinal evaluation in vitro. Am J Orthod Dentofacial Orthop. 1994;106:238-42. Dreizen S, Brown LR, Daly TE, Drane JB. Prevention of xerostomia-related dental caries in irradiated cancer patients. J Dent Res. 1977;56: 99-104. Cirano FR, Romito GA, Todescan JH. Determination of enamel and coronal dentin microhardness. Braz J Oral Sci. 2003;2(6):258-63. Moss SJ. Dental erosion. Int Dent J. 1998;48:529-39. Rytomaa I, Jarvinen V, Kanerva R, et al. Bulimia and tooth erosion. Acta Odontol Scand. 1998;56:36-40. Lackey MA, Barth J. Gastroesophageal reflux disease: a dental concern. Gen Dent. 2003;51(3):250-4. National Soft Drink Association. Soft drink facts. NSDA Web site. Available at: http://www.ameribev.org/allabout-beverage-products-manufacturing-marketing-consumption/what-america-drinks/index.aspx. Accessed May 10, 2008. Millward A, Shaw L, Smith AJ, et al. The distribution and severity of tooth wear and the relationship between erosion and dietary constituents in a group of children. Int. J. Paediatr. Dent. 1994;4:151-7. Phelan J. The erosive potential of some herbal teas. J Dent. 2003;31:241-6. Muñoz CA, Feller R, Hagland A, et al. Strengthening of tooth enamel by a remineralizing toothpaste after exposure to an acidic soft drink. J Clin Dent. 1999;10(1 spec. no.):17-21. Hooper S, West NX, Pickles MJ, et al. Investigation of erosion and abrasion on enamel and dentine: a model in situ using toothpastes of different abrasivity. J Clin Periodontol. 2003;30(9):802-808. Hunter ML, Addy M, Pickles MJ, et al. The role of toothpastes and toothbrushes in the aetiology of tooth wear. Int. Dent. J. 52: 399-405, 2002. Joyston-Bechal S, Hayes K, Davenport ES, Hardie JM. Caries incidence, mutans streptococci and lactobacilli in irradiated patients during a 12-month preventive programme using chlorhexidine and fluoride. Caries Res. 1992;26(5):384-90. Katz S. The use of fluoride and chlorhexidine for the prevention of radiation caries. J Am Dent Assoc. 1982;104(2):164-70. Sintes JL, Escalante C, Stewart B, McCool JJ, Garcia L, Volpe AR, Triol C. Enhanced anticaries efficacy of a 0.243% sodium fluoride/10% xylitol/silica dentifrice: 3-year clinical results. Am J Dent. 1995;8(5):231-5. www.ineedce.com 50 Harinho VCC, Higgins JPT, Logan S, et al. Fluoride toothpastes for preventing dental caries in children and adolescents (Cochrane Review); the Cochrane Library, Issue 2. New York:John Wiley and Sons;2004. 51 Gedalia I, Ionat-Bendat D, Ben-Mosheh S, et al. Tooth enamel softening with a cola type drink and rehardening with hard cheese or stimulated saliva in situ. J Oral Rehab. 1991;18:501-6. 52 Tung MS, Eichmiller FC. Amorphous calcium phosphates for tooth mineralization. Comp Contin Ed Dent. 2004;25(9S1):9-13. 53 Tung MS, Hwang J, Malerman R, McHale WA. Reactivity of varnish containing calcium, phosphate and fluoride salts. J Dent Res. 2007; 86(spec iss)A0:1078. 54 Final Report: Sound enamel fluoride uptake study. 2007;Study 07-107. Dental Products Testing, Indiana University Emerging Technologies Center. 55 Schemehorn BR, Wood GD, Winston AE. Laboratory enamel solubility reduction and fluoride uptake from enamel on dentifrice. J Clin Dent. 1999;10(1 Spec No):9-12. 56 Muñoz CA, Feller R, Haglund A, Triol CW, Winston AE. Strengthening of tooth enamel by a remineralizing toothpaste after exposure to an acidic soft drink. J Clin Dent. 1999;10(1 Spec No):17-21. Author Profile Dr. Fiona M. Collins, MBA, MA Dr. Fiona M. Collins has 13 years of clinical experience as a general dentist, and has held positions in professional marketing, education and training, and professional relations. She has authored and given CE courses to dental professionals and students in the US and Canada, and consulted on market research and opportunity assessment projects. Dr. Collins is a past-member of the Academy of General Dentistry Foundation Strategy Board and has been a member of the British Dental Association, the Dutch Dental Association, the American Dental Association and the International Association of Dental Research. Dr. Collins holds a dental degree from Glasgow University and an MBA and MA from Boston University. Disclaimer The author(s) of this course has/have no commercial ties with the sponsors or the providers of the unrestricted educational grant for this course. Reader Feedback We encourage your comments on this or any PennWell course. For your convenience, an online feedback form is available at www.ineedce.com. 9 Questions 1.Fully developed teeth have a finite quantity and depth of enamel and cementum. 11. The cleaning ability of sodium bicarbonate dentifrices is less than other dentifrices due to its low abrasivity. 2.As enamel loses its initial surface texture, this can cause _________. 12. Sodium bicarbonate _________. a. True b. False a. reduction in light refraction b. reduction in light reflection c. the tooth to appear darker d. all of the above 3.Intrinsic staining _________. a. is stain present inside the tooth b. only affects the outside of the tooth c. can result from stain penetrating into the dentin d. a and c 4.Intrinsic discoloration and staining has the potential to be treated chemically and/or mechanically. a. True b. False 5. Extrinsic stain can be removed and/or lifted using _________. a. hydrogen peroxide b. abrasion c. chelation d. all of the above 6.Dentifrices containing no abrasive have been found to result in _________. a. stain accumulation b. more abrasion c. patient noncompliance with oral hygiene d. a and c 7._________ have been used to gently remove stain and simultaneously help impart a polish that gives the appear ance of whiter teeth. a. Dentifrice abrasives with large, rounded particles b. Dentifrice abrasives with fine, rounded particles c. Dentifrice abrasives with fine, octagonal particles d. none of the above 8.The discoloration of nonvital teeth can only be treated by prosthetic options (crowns or veneers). a. True b. False 9.A dentifrice with high cleaning power and low abrasivity will minimize the risk of abrasion while maximizing cleaning efficacy. a. True b. False 10. The RDA of sodium bicarbonate ranges from _________ compared to an RDA of _________ in typical dentifrices. a. 20-30, 60-80 b. 30-40, 70-110 c. 35-50, 80-200 d. 50-60, 90-150 10 a. True b. False a. helps lift stain from the surface of the tooth b. cleans through both physical and chemical action c. has been shown to reduce stain in studies d. all of the above 13. Available calcium and phosphate technologies include _________. a. ACP b. Novamin c. CPP-ACP d. all of the above 14. CPP-ACP (Recaldent) is _________. a. bioavailable b. derived from sheep’s milk c. releases calcium and phosphate for remin eralization d. a and c 15. ACP consists of _________. a. an unstructured form of calcium phosphate molecules b. an unstructured form of calcium polysorbate molecules c. an unstructured form of carbinic phosphate molecules d. none of the above 16. Filling in surface defects changes the light dispersion, which can improve esthetics. a. True b. False 17. A double-blind randomized trial assessing twice-daily use of a fluoride dentifrice with ACP found that _________. a. its use improved enamel gloss b. its use decreased surface roughness c. its use gave identical results to the control dentifrice d. a and b 18. Novamin-containing dentifrice have found it to have a whitening effect. a. True b. False 19. Carbonates have been shown to control the precipitation of ACP onto tooth surfaces. a. True b. False 20. _________ causes of loss of tooth structure over time. a. Abrasion b. Erosion c. Caries d. all of the above 21. Hydroxyapatite crystals are stable at _________ . a. a pH of 3 b. a pH of 4 c. a pH of 5 d. a pH of 7 22. Erosion results in _________. a. surface loss b. the tooth losing its luster and glossy appearance c. increased susceptibility to demineralization and abrasion d. all of the above 23. _________ has been used therapeuti cally to help prevent caries. a. Fluoride b. Chlorhexidine gluconate c. Xylitol d. all of the above 24. Supersaturation of calcium and phos phate adjacent to the tooth at low pH helps prevent dissolution of crystals. a. True b. False 25. In vitro testing has found that the presence of ACP can increase fluoride bioavailability and uptake in products delivering ACP. a. True b. False 26. At a given pH, ACP _________. a. is more soluble than hydroxyapatite crystals b. will supersaturate the area with calcium and phosphate ions c. will supersaturate the area with calcium and silicate ions d. a and b 27.Conditions that discourage demin eralization and favor remineralization include_________. a. a reduced load of cariogenic bacteria b. the presence of calcium, phosphate and fluoride c. the use of chemotherapeutic agents d. all of the above 28. Clinically, products containing ACP can be used in-office followed by athome use as an oral care regimen. a. True b. False 29. ACP can precipitate onto the tooth as globules as shown by scanning electron microscopy. a. True b. False 30. Calcium and phosphate-containing dentifrice _________. a. is available over-the-counter b. does not require the patient to perform an extra step in home care c. helps esthetics and prevention d. all of the above www.ineedce.com ANSWER SHEET Treatment Options for Tooth Discoloration and Remineralization Name: Title: Address: E-mail: City: State: Telephone: Home ( ) Office ( Specialty: ZIP: ) Requirements for successful completion of the course and to obtain dental continuing education credits: 1) Read the entire course. 2) Complete all information above. 3) Complete answer sheets in either pen or pencil. 4) Mark only one answer for each question. 5) A score of 70% on this test will earn you 4 CE credits. 6) Complete the Course Evaluation below. 7) Make check payable to PennWell Corp. Mail completed answer sheet to Educational Objectives Academy of Dental Therapeutics and Stomatology, A Division of PennWell Corp. 1. List the etiologies for tooth discoloration. 2. List traditional therapies available for the treatment of tooth discoloration and describe calcium and phosphate technology and the clinical applications of this technology in treating and preventing discoloration of teeth. 3. List the etiologies for tooth demineralization. 4. List traditional and new therapies available for the prevention of demineralization and for remineralization, and describe how these work. P.O. Box 116, Chesterland, OH 44026 or fax to: (440) 845-3447 For immediate results, go to www.ineedce.com and click on the button “Take Tests Online.” Answer sheets can be faxed with credit card payment to (440) 845-3447, (216) 398-7922, or (216) 255-6619. P ayment of $59.00 is enclosed. (Checks and credit cards are accepted.) If paying by credit card, please complete the following: MC Visa AmEx Discover Course Evaluation Please evaluate this course by responding to the following statements, using a scale of Excellent = 5 to Poor = 0. 1. Were the individual course objectives met?Objective #1: Yes No Objective #3: Yes No Objective #2: Yes No Objective #4: Yes No Acct. Number: _______________________________ Exp. Date: _____________________ Charges on your statement will show up as PennWell 2. To what extent were the course objectives accomplished overall? 5 4 3 2 1 0 3. Please rate your personal mastery of the course objectives. 5 4 3 2 1 0 4. How would you rate the objectives and educational methods? 5 4 3 2 1 0 5. How do you rate the author’s grasp of the topic? 5 4 3 2 1 0 6. Please rate the instructor’s effectiveness. 5 4 3 2 1 0 7. Was the overall administration of the course effective? 5 4 3 2 1 0 8. Do you feel that the references were adequate? Yes No 9. Would you participate in a similar program on a different topic? Yes No 10. If any of the continuing education questions were unclear or ambiguous, please list them. ___________________________________________________________________ 11. Was there any subject matter you found confusing? Please describe. ___________________________________________________________________ ___________________________________________________________________ 12. What additional continuing dental education topics would you like to see? ___________________________________________________________________ ___________________________________________________________________ AGD Code 258, 780 PLEASE PHOTOCOPY ANSWER SHEET FOR ADDITIONAL PARTICIPANTS. AUTHOR DISCLAIMER The author(s) of this course has/have no commercial ties with the sponsors or the providers of the unrestricted educational grant for this course. SPONSOR/PROVIDER This course was made possible through an unrestricted educational grant from Church & Dwight Co., Inc. No manufacturer or third party has had any input into the development of course content. All content has been derived from references listed, and or the opinions of clinicians. Please direct all questions pertaining to PennWell or the administration of this course to Machele Galloway, 1421 S. Sheridan Rd., Tulsa, OK 74112 or [email protected]. COURSE EVALUATION and PARTICIPANT FEEDBACK We encourage participant feedback pertaining to all courses. Please be sure to complete the survey included with the course. Please e-mail all questions to: [email protected]. www.ineedce.com INSTRUCTIONS All questions should have only one answer. Grading of this examination is done manually. Participants will receive confirmation of passing by receipt of a verification form. Verification forms will be mailed within two weeks after taking an examination. EDUCATIONAL DISCLAIMER The opinions of efficacy or perceived value of any products or companies mentioned in this course and expressed herein are those of the author(s) of the course and do not necessarily reflect those of PennWell. Completing a single continuing education course does not provide enough information to give the participant the feeling that s/he is an expert in the field related to the course topic. It is a combination of many educational courses and clinical experience that allows the participant to develop skills and expertise. COURSE CREDITS/COST All participants scoring at least 70% (answering 21 or more questions correctly) on the examination will receive a verification form verifying 4 CE credits. The formal continuing education program of this sponsor is accepted by the AGD for Fellowship/Mastership credit. Please contact PennWell for current term of acceptance. Participants are urged to contact their state dental boards for continuing education requirements. PennWell is a California Provider. The California Provider number is 3274. The cost for courses ranges from $49.00 to $110.00. Many PennWell self-study courses have been approved by the Dental Assisting National Board, Inc. (DANB) and can be used by dental assistants who are DANB Certified to meet DANB’s annual continuing education requirements. To find out if this course or any other PennWell course has been approved by DANB, please contact DANB’s Recertification Department at 1-800-FOR-DANB, ext. 445. RECORD KEEPING PennWell maintains records of your successful completion of any exam. Please contact our offices for a copy of your continuing education credits report. This report, which will list all credits earned to date, will be generated and mailed to you within five business days of receipt. CANCELLATION/REFUND POLICY Any participant who is not 100% satisfied with this course can request a full refund by contacting PennWell in writing. © 2008 by the Academy of Dental Therapeutics and Stomatology, a division of PennWell REMIN0806RDH 11 ACP: the next big thing. The word on ACP is out. And, only one toothpaste combines it with fluoride. When you see all the facts, you’ll understand why we call this toothpaste ARM & HAMMER® Age Defying: • The only toothpaste delivering fluoride, ACP (amorphous calcium phosphate), and all the benefits of Baking Soda. • A highly effective way to strengthen and rebuild enamel daily and bring superior cleaning to patients. • Baking Soda and ACP formula maximizes whitening. – Our Baking Soda dissolves, penetrates and lifts stains from places other toothpastes can’t reach. – ACP restores enamel luster by filling in the tooth surface with minerals for a younger, brighter smile. Make it your recommendation today! For more information visit www.oralcarepro.com.