* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download PREVENTION AND CONTROL OF MRSA
Human cytomegalovirus wikipedia , lookup
Middle East respiratory syndrome wikipedia , lookup
Carbapenem-resistant enterobacteriaceae wikipedia , lookup
Marburg virus disease wikipedia , lookup
Neonatal infection wikipedia , lookup
Oesophagostomum wikipedia , lookup
Staphylococcus aureus wikipedia , lookup
Methicillin-resistant Staphylococcus aureus wikipedia , lookup
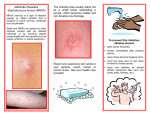
Aurora Health Care Administrative and Clinical Policy Manual Policy No: 2011 Effective Date: 09/08 Revision Dates: 09/12, 04/13 Reviewed Date: 05/15 PREVENTION AND CONTROL OF MRSA (Methicillin-resistant Staphylococcus aureus) 1. Purpose To prevent the acquisition, emergence of infection and transmission of MRSA to patients, visitors and staff. 2. Scope This policy applies to all patients, staff, and physicians at every Aurora hospital, clinic, ambulatory care center and Aurora Visiting Nurse Association (AVNA). (In this policy, ‘hospital’ refers to inpatient settings and free-standing surgery centers; ‘clinic’ refers to AMG clinics, AUWMG clinics, Aurora Advanced Healthcare clinics, Aurora Psychiatric Hospitals/Behavioral Health, and hospital-based outpatient services; ‘home health’ refers to a patient’s place of residence where services are provided by AVNA staff). 3. Definitions 3.1 MRSA – Methicillin-resistant Staphylococcus aureus MRSA is resistant to methicillin and other more common antibiotics such as oxacillin, penicillin and amoxicillin. (a) Healthcare-associated (HA-MRSA) MRSA occurs most frequently among persons in hospitals and healthcare facilities. The onset of most HA-MRSA occurs OUTSIDE the hospital, therefore is called communityonset, health care-associated MRSA. If the onset is DURING the hospital stay, it is called hospital-onset HA-MRSA. i. Community-onset Cases with at least one of the following health care risk factors: Presence of an invasive device at time of admission, History of MRSA infection or colonization, History of surgery, hospitalization, dialysis, or residence in a long-term facility in previous six (6) months preceding culture date. ii. Hospital-onset Cases with positive culture result from a site with no prior documentation of MRSA, obtained>48 hours after hospital admission. These cases might also have > one of the community-onset risk factors. (b) Community-associated (CA-MRSA): MRSA infections that occur in otherwise healthy people who have not been recently (within the six (6) months) hospitalized nor had a medical procedure (such as dialysis, surgery, catheters) are known as community-associated MRSA infections. Prevention and Control of MRSA Policy # 2011 Page 1 of 11 Aurora Health Care Administrative and Clinical Policy Manual 3.2 MSSA – Methicillin-susceptible Staphylococcus aureus 3.3 Infection – the condition when a pathogen has entered a body site, is multiplying and is causing clinical consequences such as fever, suppurative (purulent) wound or tissue destruction. 3.4 Colonization – the condition when the pathogen is present in or on a body site but where no symptoms or clinical manifestation of illness or infection are evident; the presence of bacteria without tissue invasion or damage. 3.5 Decolonization – treatment of colonized patients with antibiotics or other measures to eradicate the organism from the site of colonization (skin and mucous membranes). 3.6 Hospitalization - A patient is considered to have a history of ‘hospitalization’ if their hospital stay was longer than 24 hours duration. 3.7 MRSA PCR (MRSASC)- Rapid, qualitative molecular-based assay for the direct detection of MRSA.. This is the test that should be ordered for routine screening of MRSA carriers. (Note: the MRSA PCR may remain (falsely) positive for 2-4 weeks after treatment for MRSA due to detection of non-viable MRSA.) 3.8 Staph Aureus Screen with MRSA (SAMRSC)- This test identifies the presence of both MSSA and MRSA. It is a PCR reaction where more than one primer set is included in the reaction pool, allowing multiple DNA targets to be amplified and detected in a single reaction tube. It is more expensive than the MRSASC. The SAMRSC is for screening pre-surgical patients for both types of Staphylococcus. This test should not be routinely used for screening inpatients. 3.9 MRSA culture (CMRSA)- Culture performed on selective and differential medium for direct detection of MRSA. This test should not be used for routine screening because the PCR assay is 10-15% more sensitive and the turnaround time of the PCR assay is half that of the culture. This is the test for ‘test of cure’ if the patient has been treated in past 4 weeks for MRSA. 3.10 Microbiological clearance – this is dependent on laboratory testing. Aurora Health Care will follow guidelines for discontinuation of precautions as described in section 6.2 Discontinuation of Precautions. 4. Categories of Patients who require MRSA Screening In the hospital setting, the following should be applied for all patients who are admitted. In the clinic and home care settings, the following applies when the patient’s condition warrants consideration of MRSA colonization would increase the patient’s risk; (patient history consistent with MRSA). (a) History of MRSA (infection or colonization): A patient is considered to have a positive history of MRSA if any of the following are identified: i. Previous positive lab test (PCR or culture) from any lab ii. Patient has a self-reported history of MRSA iii. Documentation in the medical record of MRSA history. (b) High Risk Patient: These patients have no previous history of MRSA (infection or colonization) but have one or more of the following risk factors for MRSA: Prevention and Control of MRSA Policy # 2011 Page 2 of 11 Aurora Health Care Administrative and Clinical Policy Manual i. ii. Recent hospitalization, including transfers, within the prior 6 months. Admission to the ICU, including direct admits and transfers, if screen has not been completed at any previous time during patient’s current stay. iii. Patient in a long term care facility, nursing home, community based residential facility within the prior 6 months. iv. Dialysis patient v.. Patient in a correctional facility, within the prior 6 months. (c) Pre-surgical patients i. Patients undergoing cardiothoracic surgery or orthopedic procedures with hardware implantation, their risk factors, and the type of procedure should be evaluated by their physician to determine if screening, decolonization and/or prophylaxis is strongly recommended. Ideally, the MSSA/MRSA lab testing is done PRIOR to hospital admission, allowing adequate time for decolonization if necessary. Note: The overall benefits of routine screening of orthopedic patients is still being studied, but many hospitals have reported a decline in their infection rates after implementing MRSA screening programs. There is stronger evidence for screening patients for MSSA/MRSA who are undergoing cardiothoracic surgery. 5. Policy 5.1. A physician order is required for all laboratory testing for MRSA. 5.2. Patients who require screening are defined in Section 4. The type of test to use and when to screen depends on the setting of the patient encounter and the risk status of the patient. (see Table 1). 5.3. Staff will implement isolation precautions based on the clinical setting, the patient’s history and physical condition, and current MRSA status. (See Table 2) 5.4. In the inpatient setting, Contact Precautions will be followed with all patients who are known to be colonized or infected with MRSA. This includes pregnant women who are hospitalized for observation or at the time of delivery. Hospitalized patients with a history of MRSA are placed in immediate Contact Precautions until their current MRSA status is confirmed. 5.5. Per physician discretion, patients undergoing cardiothoracic surgery or orthopedic procedures with hardware implantation will be evaluated for MSSA/MRSA and complete lab testing and decolonization prior to hospital admission. 5.6. Patients that are placed on Contact Precautions during an episode of care (i.e., during a hospital stay, clinic visit) may be removed from Contact Precautions when laboratory testing identifies microbiologic clearance. 5.7. Patients who are identified as infected with MRSA may be treated in consultation with an Infectious disease physician. 5.8. Patients who have MRSA will have their status documented in their medical record. 5.9. A patient’s MRSA history may be removed only by Infection Prevention or Infectious Disease physician. Follow procedures outlined in section 6.4. Prevention and Control of MRSA Policy # 2011 Page 3 of 11 Aurora Health Care Administrative and Clinical Policy Manual Table 1. Procedures for MRSA Based on Setting and Type of Lab Test Setting When Hospital • Test immediately upon admission if meets screening criteria (see Section 4.) • Based on patient condition and physician discretion, high risk patients may be rescreened for MRSA 7 days after admission, even if their initial screening test was negative. Lab Test PCR—nares only (MRSASC) Pre-surgical Patient • Test pre-operatively, either in the outpatient setting or during the hospitalization PER PHYSICIAN DISCRETION Multiplex PCR (SAMRSC) for MRSA/MSSA is recommended for selected pre-surgical patients Clinic and Home Health, including APH/Behavioral Health • Test when the patient’s condition, reason for visit or planned surgical procedure warrants the identification of the patient’s MRSA status PCR—nares only (MRSASC) Additional/Alternative Lab Tests Per Physician Discretion* If patient has completed treatment for MRSA (infection OR colonization) within the previous 4 weeks, the PCR test may not be valid. Culture of the nares may be an alternative screening test. Culture may be used as an alternative screening test; culture has a longer turn-around time for results and may be less costly, therefore is an option for outpatient screening of pre-surgical patients. *Additional sites that may be considered for testing if indicated: peri-rectal, axilla / groin, any existing wounds, vascular catheter insertion sites, or sites that were previously positive for MRSA. Culture is the appropriate lab test for these sites. Prevention and Control of MRSA Policy # 2011 Page 4 of 11 Aurora Health Care Administrative and Clinical Policy Manual 6. Procedures 6.1. Implement Infection Control Measures Appropriate infection control measures reduce the risk of transmission of MRSA within the healthcare setting. The selection of infection control precautions depends on the clinical setting, the patient’s history and physical condition, and current MRSA status. (a) Types of Precautions (see site Infection Control manual for complete description of precaution strategies) i. Standard Precautions- follow with all patient encounters in all settings ii. Contact Precautions – see Policy statements 3, 4, and 6. iii. The patient and family will be educated (i.e., FYWB) regarding MRSA and isolation precautions. After education occurs, it will be documented in the patient’s chart and include that the patient’s and family members’ verbalized understanding. (b) Hand Hygiene. Please see Hand Hygiene Policy #183. (c) Special settings: Newborn Nursery i. Infants born to mothers infected or colonized with MRSA should remain in mother’s room as much as possible. ii. If it is necessary for infant to leave mother’s room, Contact precautions should be followed in the Newborn nursery and the infant physically separated from any other infants in the nursery. (d) Special settings: Neonatal ICU i. Infants born to mothers infected or colonized with MRSA residing in the Neonatal ICU should be placed in Contact Precautions. ii. Infants born to mothers infected or colonized with MRSA should be allowed usual visits and be allowed to breast feed and participate in Kangaroo Care as medical condition allows. These infants should remain under isolation precautions while in the nursery. iii. NICU may perform a risk assessment to determine need for surveillance culturing of their patients. iv. Caregivers who provide nursery care to multiple NICU patients (medical staff, respiratory therapy, developmental therapists, and radiology techs) should cluster work activities and minimize movement between isolation areas and the rest of the nurseries. Whenever possible, the infant in isolation should be examined/treated last. v. Infants with positive MRSA cultures should be moved to an isolation room unless other factors prohibit this. If use of isolation room is not feasible, an isolation area may be set up with screens. The isolation area should contain the following: • Contact Precautions sign clearly visible to all • A container for regular trash • A container for red bag waste • PPE (gowns, gloves, masks, eye protection Prevention and Control of MRSA Policy # 2011 Page 5 of 11 Aurora Health Care Administrative and Clinical Policy Manual vi. Cohorting of MRSA positive infants and their supplies should be implemented with dedicated nurse caregivers as much as possible. vii. Multiple births with discordant MRSA (i.e. one infant is MRSA positive, other infant is MRSA negative) status in Neonatal ICU - Parents visiting multiple infants with discordant MRSA status should visit the non-colonized infant first, while following hand hygiene and gowning procedures per unit policy viii. Management of expressed breast milk. Breast milk obtained from MRSA positive mothers with active mastitis should be discarded. Good hand hygiene should be encouraged in communal pumping areas and pumps cleaned routinely. ix. Attempts to “decolonize” neonatal/peripartum patients with topical and/or systemic antibiotics are discouraged except in an outbreak situation. . 6.2. Discontinuation of Isolation Precautions One of the following criteria must be met to remove a patient from Contact Precautions: (a) Hospitalized patients with a history of MRSA: after the results of their admission screening test (i.e., PCR) is reported as NEGATIVE (b) Hospitalized or Clinic patients that have been treated for MRSA in the previous 4 weeks and meet the following: i. Patient has been off antibiotic therapy for at least 48 hours AND ii. Two consecutive sets of negative cultures of all previously positive sites at least 24 hours apart have been obtained. • • If one or both of the cultures are positive, the patient must remain in isolation and further evaluation may be warranted. Sufficient confirmation of the above treatment and microbiologic clearance has been obtained. Laboratory testing to confirm microbiologic clearance may be completed on an outpatient basis, prior to a hospital admission. (c) Special settings: Neonatal ICU: same as above 6.3. Decolonization of patients (a) Routine decolonization of all patients colonized with MRSA is not recommended. (b) However, specific patient condition or reason for hospital admission or outpatient visit may warrant decolonization to prevent progression to infection. Current literature supports the decolonization for the following categories of patients: • Dialysis patients • Patients with recurrent S. aureus infections • Certain surgical procedures such as cardiothoracic and orthopedic procedures. (c) Consultation with an infectious disease physician may be appropriate for determining treatment course, selection of medications and duration of treatment. Prevention and Control of MRSA Policy # 2011 Page 6 of 11 Aurora Health Care Administrative and Clinical Policy Manual 6.4 Removal of MRSA history (a) A patient’s MRSA history may be removed only by Infection Prevention or Infectious Disease physician if ALL of the following criteria are met: i. ii. iii. iv. No high risk conditions (as specified in Section 4.) No active MRSA infection No positive MRSA cultures in the last 6 months. Documented PCR negative in previous 6 months while off antibiotics (b) If patient does not meet all criteria above, may consider consultation with an Infectious Disease physician for further consideration of removal. Prevention and Control of MRSA Policy # 2011 Page 7 of 11 Aurora Health Care Administrative and Clinical Policy Manual Table 2. Infection Control Measures to Prevent and Control MRSA HOSPITAL (includes FREE-STANDING SURGERY CENTER) Setting and Type Type of When to Initiate Additional of Patient Precautions Infection Control Measures High risk patient Standard Screen for MRSA precautions History of MRSA Contact Immediately upon Precautions admission, prior to any lab tests performed or results returned Patient with positive MRSA test Contact Precautions All Patients undergoing a splash-generating procedure OR caring for patients with a potential for projectile secretions Droplet Precautions (masks) Immediately after laboratory confirmation of MRSA colonization or infection During procedure Removal of Precautions If all initial screening tests are negative for MRSA OR Documentation is provided indicating appropriate treatment and microbiologic clearance. Patient has received appropriate treatment and microbiologic clearance After splashgenerating procedure is completed OR when there is no potential for projectile secretions CLINIC SETTING (i.e., AMG, AUWMG and Aurora Advanced Clinics) and HOSPITAL-BASED OUTPATIENT SERVICES including APH/Behavioral Health Setting and Type Type of When to Initiate Additional of Patient Precautions Infection Control Measures High risk patient Standard • Use precautions disposable equipment, when possible • Follow site policies regarding disinfecting re-usable equipment (i.e., BP cuff, tympanic thermometer), and environmental surfaces prior to next room use. Prevention and Control of MRSA Policy # 2011 Page 8 of 11 Removal of Precautions Aurora Health Care Administrative and Clinical Policy Manual History of MRSA Contact Precautions Initiate if patient has: • • • Uncovered wounds Incontinent Hygiene concerns that may expose the environment to secretions/ bodily fluids Immediately place patient in private exam room; avoid wait time in general reception area After risk of exposure is resolved. Additional Infection Control Measures • Limit the amount of equipment carried into the home • Use disposable equipment, when possible • Follow policies regarding disinfecting re-usable equipment (BP cuff, tympanic thermometer) Removal of Precautions HOME HEALTH Setting and Type of Patient Type of Precautions High risk patient Standard Precautions History of MRSA Contact Precautions When to Initiate Initiate if patient has: • • • Prevention and Control of MRSA Policy # 2011 Page 9 of 11 Uncovered wounds Incontinent Hygiene concerns that may expose the environment to secretions/ bodily fluids After risk of exposure is resolved. Aurora Health Care Administrative and Clinical Policy Manual Cross References: References: Calfee DP, et al. Strategies to Prevent Transmission of Methicillin-Resistant Staphylococcus aureus in Acute Care Hospitals. Inf Control and Hosp Epi. 2008 Sept 16: 29(S1) S62-S80. Centers for Disease Control and Prevention (CDC). www.cdc.gov CDC: Healthcare Infection Control Practices Advisory Committee (HICPAC) (2006) Management of Multidrug-Resistant Organisms in Healthcare Settings, 2006. HICPAC Guidelines Centers for Disease Control and Prevention (CDC). Methicillin-resistant staphylococcus aureus infections among competitive sports participants--Colorado, Indiana, Pennsylvania, and Los Angeles County, 2000-2003. MMWR Morb Mortal Wkly Rep. 2003 Aug 22;52(33):793-5. Centers for Disease Control and Prevention (CDC). Methicillin-resistant Staphylococcus aureus infections in correctional facilities---Georgia, California, and Texas, 2001-2003. MMWR Morb Mortal Wkly Rep. 2003 Oct 17;52(41):992-6. Diekema D. et al. Current Practice in Staphylococcus aureus Screening and Decolonization Inf Control and Hosp Epi 2011 Oct; 32(10): 1042-1044. Gastelum DT, Dassey D, Mascola L, Yasuda L. Transmission of community-associated methicillin-resistant Staphylococcus aureus from breast milk in the neonatal intensive care unit. Ped Infect Dis J 2005; 24: 1122-4. Gerber SL, Jones RC, Scott MV, Price JS, Dworkin MS, Filippel MB et al. Management of outbreaks of Methicillin-resistant Staphylococcus aureus infection in the Neonatal intensive care unit; a consensus statement. Infect Control Hosp Epidemiol 2006: 27: 139-45. Huskins WC, et al. Intervention to Reduce Transmission of Resistant Bacteria in Intensive Care. N Eng J Med 2011 April; 364(15): 1407-1418. Kazakova SV, et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med. 2005 Feb 3;352(5):468-75. Kellie Susan M. Methicillin-resistant Staphylococcus aureus (MRSA) in pregnancy: Epidemiology, clinical syndromes, management, prevention, and infection control in peripartum and post-partum periods. http://www.antimicrobe.org/b237index.asp Klevens RM, et al. Invasive Methicillin-Resistant Staphylococcus aureus Infections in the United States. JAMA. 2007;298:1763-1771. Liu C, et al. Clinical Practice Guidelines by the Infectious Diseases Society of America for the Treatment of Methicillin-Resistant Staphylococcus Aureus Infections in Adults and Children. Clinical Infectious Disease 2011: 52: 1-38. Morel AS, Wu F, Della-Latta P, Cronquist A, Rubenstein D, Saiman L. Nosocomial transmission of methicillin-resistant Staphylococcus aureus from a mother to her preterm quadruplet infants. AJIC 2002; 30: 170-173. Prevention and Control of MRSA Policy # 2011 Page 10 of 11 Aurora Health Care Administrative and Clinical Policy Manual Regev-Yochay G, Rubinstein E, Barzilai A, Carmeli Y, Kuint J, et al. Methicillin-resistant Staphylococcus aureus in Neonatal Intensive Care Unit. Emerging Infectious Diseases, 11(3): 453-6. Safdar N, Maki DG. The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant Staphylococcus aureus, enterococcus, gramnegative bacilli, Clostridium difficile, and Candida. Ann Intern Med. 2002 Jun 4;136(11):834-44. Siegel, J., Rhinehart E., Jackson M., Chiarello L & the Healthcare Infection Control Practices Advisory Committee, 2006. Management of Multidrug Resistant Organisms in Healthcare Settings, 2006 Turabelidze G, et al. Personal Hygiene and Methicillin-resistant Staphylococcus aureus Infection Emerging Infectious Diseases. Available at www.cdc.gov/eid; Vol. 12, No. 3, March 2006 Weber SG, et al. Fluoroquinolones and the risk for methicillin-resistant Staphylococcus aureus in hospitalized patients. Emerg Infect Dis. 2003 Nov;9(11):141522. Weber SG, et al. Legislative Mandates for the Use of Active Surveillance Cultures to Screen for Methicillin- Resistant Staphylococcus aureus and Vancomycin-Resistant Enterococci: Position Statement From the Joint ShEA and APIC Task Force. Infect Control Hosp Epidemiol 2007;28:249-260 Owner: System Infection Prevention Review Dates: 09/12, 05/15 For more information about MRSA, go to the Care Management/Patient Safety iConnect site Prevention and Control of MRSA Policy # 2011 Page 11 of 11