* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download view article - Portland Veterinary Specialists
Management of acute coronary syndrome wikipedia , lookup
Myocardial infarction wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Cardiac surgery wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Atrial fibrillation wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
John M M G M U Q
PVM. DipWVIM
Clinical Situation in Cardiology
iCjlI\l»|n^v)
Laor* E, Iblton
DVM
Several episodes of collapse
MASSACHUSETTS
\ HTF.RJ N AR*i
REFERRAL
HOSPITAL
CLINICAL CASE:
A 12-year-old spayed female Shih Tzu, weighing 10.3 kg, presented to the
Cardiology Service because of a three-week history of lethargy, exercise
intolerance, and several episodes of collapse. She had a history of mild,
asymptomatic, myxomatous mitral valve disease with minimal left atrial
enlargement but no other significant medical problems. She was receiving no
therapy for her valvular disease. The collapse episodes consisted of slowing
down dramatically, becoming unsteady on her feet, and then collapsing. She
would recover within several seconds and act as if nothing had happened.
During her physical examination (PE), her heart rate was 110 beats per
minute (bpm) and her rhythm was irregular with several one-second pauses.
A grade 2/6 systolic left apical murmur was ausculted, consistent with
previous examination findings. No other abnormalities were noted on
thoracic auscultation. Her pulses were strong and synchronous and her
mucous membranes were pink with a capillary refill time of less than 2
seconds. Her neurologic examination was normal. Screening bloodwork
showed no abnormalities. Her packed cell volume (PCV) was 4 2 % and her
total solids were 7.2 mg/dl, both of which are considered normal. Thoracic
radiographs were taken (Figure 1), which demonstrate mild cardiomegaly
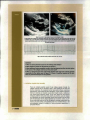
but no other significant thoracic abnormalities. An echocardiogram was
performed (Figure 2), which demonstrated a mildly leaky mitral valve with
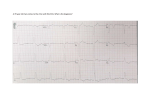
mild left atrial and left ventricular enlargement. An electrocardiogram (ECG)
was performed as well (Figure 3).
Woburo, MA 01801
Figure i (a) and (b). Thoracic radiographs of the patient.
Right lateral (Fig. 1 (a) left image) and DV (Fig. 1 (b) right
image) thoracic radiographs. Note mild cardiomegaly and
mild left atrial enlargement (arrows).
considta • 1
ANSWERS
Figure 2 (a) and (b). 20 right parasternal long-axis echocardiography images.
The left ventricle (LV), left atrium (LA), interventricular septum (IVS). left ventricular free wall (FW), and anterior leaflet of the mitral valve (MV)
are labelled in Fig 2 (a) left Note mild to moderate left ventricular and left atrial enlargement and the thickened mitral valve. Fig. 2 (b) right
shows the same structures as Fig 2 (a) left but this image is in systole and demonstrates mild to moderate mitral regurgitation (MR) with
mild mitral valve prolapse.
1
Br
•
33
; LA,"
T » -
Figure 3. ECG from current visit (lead II, 25 mm/sec, imV • 10 mm).
Questions
1. What are some potential causes for the lethargy and collapse?
2. What rhythm is present in the ECG seen in Figure 3 ?
3. What additional testing modalities could be used to diagnose/confirm that this rhythm
is the cause of the collapse and how are they best used?
4. What is a diagnostic test that could reveal whether high vagal tone could be partially
responsible for the rhythm seen in Figure 3? If there is a positive response to this test,
w h a t are potential therapies for this patient?
1 - POTENTIAL CAUSES FOR COLLAPSE
There are multiple potential causes of any collapse episode. Broadly, the
collapses could be due to neurologic causes, causes related to the heart that
directly compromise the pumping function (pericardial effusion, pulmonary
hypertension), causes related to heart rate/rhythm, causes related to blood loss
(internal or external), or metabolic causes (which might include an Addisonian
crisis among other potential causes). In this case, blood loss and common
metabolic problems were given lower priority as potential causes of collapse
since Woodwork was normal. Neurologic causes were thought to be less likely,
given the description of the collapse episodes (no tonic/clonic motion, no preor post-ictal phase) and the fact that the patient's neurologic examination was
normal. Structural cardiac pump problems were not considered likely, as the
echocardiogram showed no evidence of either pulmonary hypertension or
pericardial effusion.
2 • consutta
Collapse can be caused by several different types of syncope. Tussive syncope
("cough drop" syncope) is a common cause of syncope, which typically occurs
in small breed dogs with tracheal collapse, chronic pulmonary disease, and/or
brachycephalic syndrome. Episodes of syncope typically occur during or
immediately after coughing. One mechanism proposed to explain "cough
drop" syncope is that coughing causes an increase in intrathoracic pressure,
which causes a transient increase in intracranial pressure with subsequent
diminished cerebral blood flow. Coughing may also cause a decrease in cardiac
venous return and, therefore, limit cardiac output. Alternatively, coughing
causes vagal stimulation to the heart and blood vessels, resulting in
bradycardia and hypotension (i.e. reflex cardiac slowing +/- peripheral
vasodilation similar to neurocardiogenic syncope). In this patient, tussive
syncope was thought to be unlikely, as coughing was not a feature of the
collapse episodes. Autonomic dysfunction (vasodepressor or neurocardiogenic
syncope vs. reflex-mediated syncope) is the result of withdrawal in sympathetic
tone with a concomitant increase in parasympathetic activity. The result is
hypotension and bradycardia causing a decrease in cerebral blood flow with
subsequent syncope. It is considered an abnormal and inappropriate activation
of the baroreceptor reflex, which in humans typically occurs in hypovolemic or
hypotensive patients. In dogs, these episodes are typically brought on by
exertion or excitement and it is not apparent that dehydration is a predisposing
factor. Neurocardiogenic syncope can occur in normal healthy animals or in
animals with cardiac disease. It commonly occurs in small breed dogs with
advanced mitral valve disease. In these patients a hyperdynamic left ventricle
may occur with severe mitral regurgitation. This may mimic the hypovolemic
human patient in that high sympathetic tone will increase cardiac contractility
and, with concurrent severe mitral regurgitation, will cause an "empty
ventricle". Sympathetic withdrawal then occurs in these patients, resulting in
vasodilation, increased vagal activity, and subsequent bradycardia and collapse.
Neither cause of autonomic dysfunction was thought to be a cause of syncope
in this patient.
ANSWERS
In this patient, arrhythmia was detected during the PE and was consistent with
several types of arrhythmias that can cause collapse when the severity is
sufficient.
2 - THE RHYTHM PRESENT IN THE ECG SEEN IN FIGURE 3
B
t
i
1
• ' t-
T
•
Figure 3 (labelled). ECG from current visit (lead II, 25 mm/sec, lmV - 1 0 mm). Arrows indicate P waves that are blocked (no QRS complex
follows the P waves). The arrowhead demonstrates a ventricular escape beat. The 'A' distance Is a period of sinus arrest of 3.6 seconds.
Complex "8" is a premature supraventricular complex.
The rhythm present is second-degree Mobitz type II atrioventricular (AV) block
with a period of sinus arrest, a premature supraventricular beat, and a
ventricular escape beat. Atrioventricular block is characterized by either the
delay or complete extinguishing of an electrical impulse through the AV node
and is generally due to AV-node injury or fibrosis. Normal sinus beats originate
in the sinus node, travel through the atria via Bachmann's bundle and reach
the AV node. The depolarization of the atria that this causes is seen on the
ECG as a P wave. The impulse travels through the AV node at a somewhat
slower speed, allowing the ventricles to be filled by the contracting atria prior
consutta • 3
to ventricular depolarization. In first-degree AV block, the atrial impulse is
abnormally delayed, resulting in a prolonged P-R interval. In second-degree AV
block, one or more of the atrial impulses do not pass through the AV node.
There are two types of second-degree AV block - Mobitz type I and Mobitz
type II. In Mobitz type I second-degree AV block, there is successive
prolongation of the P-R interval with each beat until one P wave is blocked
completely. First-degree AV block and Mobitz type I AV block do not cause
clinical signs and, generally, are not treated. In Mobitz type II second-degree
AV block, prolongation of the P-R interval does not occur prior to the P wave
being blocked. Mobitz type II second-degree AV block can be described by the
ratio of total P waves to conducted P waves. As an example, when only every
9th P wave is conducted, it is called 9:1 second-degree AV block (Figure 4).
Mobitz type II second-degree AV block can cause clinical signs depending on
the severity of the AV block.
ANSWERS
t•i
H
^
^
^
^
Figure 4. Example of 9:1 second-degree AV block (lead II, 25 mm/sec, ImV - 1 0 mm). Single-headed arrows indicate P
waves and arrowheads indicate QRS complexes. Double-headed arrows demonstrate the P-R interval from the conducted
(ninth) P waves.
In third-degree AV block, none of the P waves are conducted through the AV
node (Figure 5). Ventricular depolarization occurs secondary to development
of an "escape rhythm" that emanates from pacemaker cells either in the more
distal part of the AV junction (the His bundle) or in the ventricles. When the
escape rhythm emanates from the AV node or Bundle of His, the QRS
complexes are narrow with an escape rate of 40 to 60 bpm. When the escape
pacemaker is in the ventricular myocardium, the QRS complexes appear wide
and bizarre and the escape rate is 20 to 40 beats/minute. Third-degree AV
block is more common in older animals, but can occur at any age. Patients may
present for lethargy, exercise intolerance, or collapse. Some animals will
develop signs of congestive heart failure causing dyspnoea, tachypnoea,
coughing, or ascites. Physical exam findings reveal bradycardia (HR typically <
50 beats/minute in dog). Intermittent jugular pulsations called "cannon a
waves" may be present, which are caused by intermittent right atrial
contraction against a closed tricuspid valve. The first heart sound may vary in
intensity secondary to variability in the end-diastolic ventricular volume, a
phenomenon called "bruit de canon". Some animals will also have signs of
low-output failure (i.e. forward heart failure) or congestive heart failure. At
times it is difficult to distinguish between high-grade second-degree AV block
and third-degree AV block and calling it advanced AV block suffices, as it
describes both conditions.
ii
t
• t
• M • t y
•*—M
T
I \ •
Figure 5. Third-degree AV block (tead II, 25 mm/sec, ImV • 10 mm). Single-headed arrows indicate P waves.
Double-headed arrows demonstrate the P-R interval. Note the variable P-R interval, which Is consistent with
third-degree (complete) AV block.
4 •consutta
i
Sinus arrest is defined as a failure of a normal impulse to be formed in the
sinus node owing to a depression of automaticity in the sinus node. This is
often difficult to distinguish from sinus node block, in which the impulse is
formed normally but a conduction abnormality exists, preventing the impulse
from reaching the rest of the heart. Both result in pauses where no P wave is
seen. In sinus block, the R-R length of the pause is an exact multiple of the R-R
intervals preceding the pause, whereas in sinus arrest the pause can be any
length greater than 2 R-R intervals. In practice it is often quite difficult, and
clinically irrelevant, to distinguish between the two underlying causes of lack
of P wave formation and the resultant ECG is simply called sinus arrest.
3 - ADDITIONAL TESTING MODALITIES THAT COULD CONFIRM THAT THE CAUSE OF
THE COLLAPSE EPISODES WAS DUE TO THE RHYTHM DEMONSTRATED A N D HOW
THE TEST SYSTEMS ARE BEST USED.
A 24-hour ambulatory ECG monitor (Holter monitor) or an event recorder
could be used to demonstrate that the rhythms observed in the room second-degree AV block or sinus arrest - were the cause of the collapsing
episodes. A Holter monitor records 24-72 hours of continuous ECG data.
These data are recorded either on a cassette tape or digitally. The patient has
5-7 electrodes placed on their skin and then carries the monitor for the
duration of the testing period (Figure 6). The data from the monitor are then
analysed and a catalogue of the rhythm is generated via a computer program.
A Holter monitor is most useful for determining the severity of the arrhythmia
present in the testing period or to determine the efficacy of the anti-arrhythmic
therapy. It is less useful for figuring out the cause of collapsing episodes that
happen relatively infrequently.
Figure 6.24-hour ambulatory
ECG monitor. Note the cassette
tape where ECG data are
stored, battery compartment,
and ECG cable. The ends of the
ECG leads are attached to selfadhering electrode patches on
either side of the thoracic
cavity at the level of the 4-6"
intercostal spaces.
In order to determine accurately whether the cause of the episode is due to the
arrhythmia seen on the Holter monitor, the patient diary needs to be
synchronized relatively precisely to the time on the Holter monitor. In contrast,
an external event loop recorder (Figure 7 (a)) has 2 electrodes and is worn for
up to one week at a time. It records continuously but does not save data until
a button is pressed on the recorder by the pet owner during an event. The
recorder then saves an ECG loop. Since the data are saved in a loop, the
recorder contains the ECG recording for several seconds prior to the button
being pushed, the ECG when the button was pushed, and the ECG for several
seconds after the button was pushed. This enables the event recorder to
"reach back in time" to capture the ECG that was most likely to be associated
with the clinical signs exhibited by the patient. These event recorders can be
worn for up to a week at a time and are most useful when the patient is having
2 or more events weekly. The final option is an implantable loop recorder
(Figure 7 (b)). This is a device that is implanted subcutaneously and can be
programmed to record all low and high heart rate events. The data are read
and programmed via an external unit (7 (c)) and can be used to determine
whether rhythm events are the cause of rare collapse events.
Figure 7 (a). External event loop recorder. Note the 2
electrodes that are attached to self-adhering electrode
patches on either side of the thorax. Electrodes are
placed over the heart in the middle of the thorax. Note
the "Record" button in the centre of the recorder. In
the event of a collapse episode, the patient's owner
presses the button and the ECG loop that was
recorded is stored. These data are then transferred
transteiephonicaliy and an ECG is generated.
Figure 7 (b). Implantable loop recorder. Note the US
dime (18 mm) placed in the picture for size reference.
The event recorder is placed subcutaneously and stays
in the patient for many months. The event recorder
can be programmed to record high and low heart rate
Incidents and a remote control device can be used by
the owner to mark events in the memory.
Figure 7 (c). Pacemaker programmer. This is used to
program the event recorder and to download
information from the implantable event recorder. The
programming head (arrow) is placed over the event
recorder while the event recorder is still in the patient
and information is transmitted from the event
recorder to the programmer for analysis.
4 - DIAGNOSTIC TEST
ANSWERS
An atropine response test can be used to determine the influence of
parasympathetic (vagal) tone on the heart rate and rhythm. Atropine is a
competitive antagonist of the muscarinic acetylcholine receptors, which acts as
a parasympatholytic and, as such, abolishes or diminishes the influence of the
vagus nerve on the heart rate. This leads to increased firing of the sinus node
and can increase conduction through the AV node, if this is being inhibited by
high vagal tone. In this test, a baseline ECG is recorded and then 0.04 mg/kg
of atropine is administered intravenously. Ten to 15 minutes later, a follow-up
ECG is recorded. If the rhythm abnormality is due to high vagal tone, the heart
rate in the follow-up ECG should be significantly faster and the abnormalities
should normalize. The result is often sinus tachycardia. In this patient, atropine
administration did result in sinus tachycardia (Figure 8).
Figure 8. ECG obtained 15 minutes after atropine administration as part of an atropine response test (lead II, 25 mm/sec ImV - 1 0 mm). The
heart rate is 150 beats per minute and the rhythm is sinus tachycardia. This indicates a positive response to atropine and demonstrates that the
arrhythmia was at least partially due to high vagal tone.
Potential therapies for this patient if there is a positive response to this test
If there is a positive response to atropine, several oral medications may be used
to help normalize the rhythm. Propatheline bromide is a quaternary
antimuscarinic antichoienergic that can be given orally three times daily at a
dose starting at 7.5 mg/patient q 8 hours. This can be increased to 30
mg/patient q 8 hours as needed. Common side-effects are dry mouth and eyes,
anxious behaviour and Gl signs. Hycosamine, an anticholinergic alkaloid, can
also be used at 0.003 to 0.006 mg/kg q 8 hours. In this patient. Propantheline
bromide was initiated at 7.5 mg q 8 hours. At a recheck examination, the
owner reported that the patient did not have any more collapse episodes and
an ECG showing intermittent Mobitz type II 2:1 AV block (Figure 9) was
recorded. No change was instituted in the therapy and the patient was
discharged with instructions to return in 1 month for a recheck ECG.
j
i:
-
* i ' I
-1
_ ;. ;
*
— i
j
r
t
1'.Z'
— j —
: i
1
4 1
pi
1
i
•ft
•
-
Figure 9. ECG obtained after 1 week of propantheline bromide therapy (lead II, 25 mm/sec, ImV • 10 mm). The heart rate is 75-150 beats per
minute and the rhythm is sinus tachycardia with Mobitz type II second4egree AV block present. Arrows represent blocked P waves.
FOLLOW-UP
The patient re-presented prior to the scheduled recheck examination with a
return of collapsing episodes. An ECG was obtained (Figure 10 (a)) that was
consistent with third-degree AV block. At the owner's request, the atropine
response test was repeated but a satisfactory response was not obtained. The
patient's heart rate rose slightly from 15-18 beats per minute to 20-25 beats
per minute, but this was not considered to be sufficient improvement to have
the patient lead a life with normal activity and not collapse (Figure 10 (b)).
consutta • 7
ANSWERS
Figure 10 (a). Third-degree AV block (lead II, 25 mm/sec, ImV • 10 mm). Red arrows denote junctional escape beats and blue arrows represent
premature ventricular complexes. Note the variable P-R interval and extremely slow ventricular rate.
t\ "
A
!i
k
Figure 10 (b). Third-degree AV block after the atropine response test (lead II, 25 mm/sec, ImV - 1 0 mm). Red arrows denote P waves "buried" in
the QRS complex. Note the variable P-R interval consistent with third-degree AV block. Note also that the atrial rate has increased and that the
junctional escape rate has increased as well. This Indicates that there was some vagal influence on the junctional escape rate.
5. What treatment options are available at this juncture?
6. What are some of the potential methods to stabilize the patient prior to surgery?
7. Describe the components of a pacemaker system and some of the potential pacemaker
modalities that could be used in this patient.
5 - TREATMENT OPTIONS AVAILABLE AT THIS JUNCTURE
In most cases of complete AV block, an underlying cause is not found and it is
therefore presumed to be secondary to idiopathic fibrosis; however, an attempt
should be made to address or diagnose any possible causes for this condition.
Potential aetiologies include congenital defects (e.g. aortic stenosis, ventricular
septal defect), infiltrative diseases (e.g. neoplasia or amyloidosis), excessive
vagal tone, bacterial endocarditis, myocardial infarction, hypothyroidism,
infection (e.g. Trypanasoma cruzi (Chagas disease) and B. Burgdorferi),
hyperkalaemia, drugs (e.g. digitalis, beta-blockers or calcium-channel
blockers). In these cases, treatment of the underlying condition should be
attempted if possible. However, in this patient, none of the above conditions
were suspected.
The goals of treatment are to restore normal cardiac output and/or resolve
congestive heart failure if this is present. Positive chronotropic medications can
be administered, such as theophylline (5-15 mg/kg PO BID of the extended
release formulation) or terbutaline (0.2 mg/kg PO BID-TID). However, response
is variable and almost always unrewarding with third-degree AV block. Chronic
treatment requires permanent artificial pacemaker implantation to resolve
clinical signs and provide a more favourable long-term prognosis. In this case,
pacemaker implantation was chosen.
6 - SOME OF THE POTENTIAL METHODS TO STABILIZE THE PATIENT PRIOR TO SURGERY
Temporary artificial pacemaker implantation may be considered in patients that are at high
risk for anaesthesia or require stabilization prior to implantation of a permanent pacemaker. This decision should be based on the stability of the patient, experience of the clinician
in pacemaker placement, and availability of equipment. There is no agreed-upon standard
8 •consutta
in veterinary medicine as to when temporary pacing should be used. Temporary pacing can
use either transvenous or transthoracic approaches, based on the clinician's preference.
Many cases that are haemodynamically stable can proceed directly to permanent pacemaker implantation. Typically some type of temporary pacing system is placed prior prepping
patients for permanent pacemaker implantation in order to control the rhythm should the
rate become even more bradycardic during induction. In this case, transthoracic pacing was
used. Transthoracic pacing uses a defibrillator with pacing capabilities and surface electrodes that are attached bilaterally over the patient's thoracic wall. General anaesthesia is used
to prevent pain associated with the electrical stimulation itself and, typically, transthoracic
pacing is only administered if the patient is not haemodynamically stable during permanent
pacemaker placement.
ANSWERS
7 - DESCRIPTION OF THE COMPONENTS OF A PACEMAKER SYSTEM AND SOME OF
THE POTENTIAL PACEMAKER MODALITIES THAT COULD BE USED IN THIS PATIENT.
The critical components of a pacing system include a pulse generator that has
been programmed appropriately and a pacing lead (Figure 11). The cardiac
pacemaker functions as an electrical circuit, whereby the pulse generator
(battery) provides electrical stimulation that travels through the pacing lead to
the myocardium and then back to the battery to form a complete circuit. All
pacemaker systems have two poles. Bipolar pacemaker leads are most
commonly used and create a smaller circuit with both poles located near the
end of the pacemaker lead. Unipolar leads have one pole located near the end
of the lead, with the other pole encompassing the metal of the pacemaker
generator. Unipolar leads therefore contain a larger circuit of electricity and
can trigger activation of surrounding musculature. Bipolar leads only trigger
stimulation of the ventricular myocardium and are less likely to pick up stray
signals from the environment
Figure 11. Pacemaker generator (GEN)
and lead (Lead). Note active fixation,
screw-in type tip (AF), and bipolar sites
(arrows).
Pacemaker modes are described using a 3- to 5-letter classification system
(Table 1). This system categorizes pacing based on the site and mode of
cardiac pacing and sensing. The first letter indicates the chamber paced, the
second letter indicates the chamber sensed, and the third position describes
the expected response to sensing. The fourth describes the programmable
features of the device. The fifth indicates whether an anti-tachyarrhythmia
function is available.
consutta* g
Pacemaker modes commonly used in small animals
!
1 Chamber(s) paced
II Chamber(s) sensed
III Response to sensing
rv Programmable functions
V • ventricle
V = ventricle
T = triggers pacing
P = simple programmable
A = atrium
A = atrium
1 = inhibits pacing
M - multiprogrammable
0 • dual chamber
D = dual chamber
0 = triggers and inhibits pacing C = communicating functions
0 = none
O-none
O = none
S*AOfV*
S = AorV*
R = rate modulating
0 = none
*S: used by some manufacturers to indicate single-chamber (A or V)
The most common pacing mode is W l in which the ventricle is paced (V);
the pacemaker senses native cardiac depolarizations in the ventricle (V) and
then inhibits pacemaker discharge d u r i n g these periods (I). W i t h
conventional W l pacing, the patient's heart rate is fixed and does not
change w i t h variable cardiac output demands of the patient. In contrast,
W I R pacing is rate responsive and uses an activity sensor that attempts to
correlate patient activity w i t h heart rate. Rate-responsive pacemakers (R)
change the pacing rate based on parameters such as blood temperature,
oxygen saturation or pH, ventilation rate, gravitation sensors, or
accelerometer functions.
The most common method of pacemaker placement uses a single-chamber
pacemaker programmed to ventricular demand ( W l or WIR) modes. The
pacing lead is inserted into the right ventricular apex through the jugular vein.
An endocardial lead is advanced into the right ventricular apex using
fluoroscopic guidance. The tip of the lead is either actively or passively
attached to the RV endocardium. After the lead is securely in place, its
proximal end is attached to the pacemaker generator. The pacemaker
generator is placed in a subcutaneous pocket dorsal to the incision over the
jugular vein. A subcutaneous tunnel is created that will pass from the jugular
vein to the generator.
Dual-lead chamber pacing (DDD, DDDR) uses a second lead placed within the
right auricular appendage, which senses and paces this chamber. DDD pacing
is beneficial in that it maintains synchrony between atrial and ventricular
contractions. It is the most commonly used pacing mode in humans. DDD
pacing has been described in veterinary patients, but is not as widely used due
to technical limitations in small animals, expense, and complication associated
with placing a separate atrial lead. This modality is particularly useful in
advanced heart block, as it allows the patient's intrinsic sinus rhythm to control
the rate of ventricular depolarization. Care must be taken to set an
appropriately high baseline heart rate, as sinus node dysfunction often
accompanies AV node dysfunction.
Single-lead synchronous VDD pacing allows maintenance of AV synchrony
without the need of two leads as in the DDD pacing system. These leads use a
proximal set of electrodes on the outer surface of the pacing lead, which are
able to detect atrial electrical activity as it is conducted through the right
atrium. The atrial electrodes do not come into direct contact with the atrial
tissue, but are sensed through "floating" atrial electrodes (Figure 12).
Following proper programming, atrial depolarization will lead to ventricular
pacing that mimics the normal AV conduction period. VDD pacing has been
shown to increase stroke volume and cardiac output as well as to decrease left
atrial size, pulmonary capillary wedge pressures, and circulating biomarkers.
Overall, VDD or DDD systems should be considered for their haemodynamic
advantage in patients with advanced atrioventricular block and normal sinus
node function.
Final Outcome
In this patient a single-chamber pacemaker in WIR mode was implanted
through the right jugular vein. The bottom rate was set at 80 bpm and the top
rate was set at 140 bpm. Placement was confirmed with post-op radiographs
(Figure 13 (a)). The patient was monitored overnight with continuous ECG
monitoring and the rate was never below 80 bpm. The patient went home the
following morning. In 10 days, the skin incision sutures were removed and an
ECG was checked (Figure 13 (b)).
Figure 13 (a) and (b). Thoracic radiographs post-pacemaker implantation.
Fig 13 (a) left: right lateral thoracic radiograph showing the pacemaker generator (Gen) within the right
lateral neck. The pacemaker lead then courses from the generator through the subcutaneous tissues, right
jugular vein (Right Jugular), cranial vena cava (CrVC),rightatrium (RA), and finally into the right ventricular
apex (RV) where the lead tip comes into contact with the right ventricular endocardium.
ANSWERS
References
Tilley LP. Analysis of common canine cardiac arrhythmias. In Tilley LP (ed):
Essentials of Canine and Feline Electrocardiography. Malvern, Lea & Febiger,
1992, 127-207.
Cote E, Ettinger SJ. Electrocardiography and Cardiac Arrhythmias. In SJ
Ettinger and Feldman EC (eds): Textbook of Veterinary Internal Medicine. St.
Louis, Elsevier Saunders, 2005, 1040-1076.
Moise NS. Diagnosis and Management of Canine Arrhythmias. In Fox PR,
Sisson D, Moise NS (eds): Textbook of Canine and Feline Cardiology.
Philadelphia, W.B. Saunders, 1999, 331-385.
Hyoscyamine Sulfate. In Plumb DC: Plumb's Veterinary Drug Handbook.
Ames, Blackwell Publishing, 2008. 469-470.
Propantheline Bromide. In Plumb DC: Plumb's Veterinary Drug Handbook.
Ames, Blackwell Publishing, 2008. 775-776.
Kittleson MD: Syncope. In Kittleson (ed):
Medicine. St Louis, Mosby, 1998 pp 495-501.
Small Animal Cardiovascular
Rush JE: Syncope and episodic weakness. In Fox PR, Sisson D, Moise NS (eds):
Textbook of Canine and Feline Cardiology. Philidelphia, Saunders, 1999, pp
446-454.
Moise NS: Pacemaker Therapy. In Fox PR, Sisson D, Moise NS (eds): Textbook
of Canine and Feline Cardiology. Philidelphia, Saunders, 1999, pp 400-425.
Petrie JP: Permanent Transvenous Cardiac Pacing: Clinical Techniques in Small
Animal Practice 20: 164-172, 2005.
Bulmer BJ et al: Physiologic VDD versus non-physiologic W l pacing in canine
third degree atrioventricular block, J Vet Intern Med 20: 257, 2006.
Oyama, MA, Sisson D: Permanent Cardiac Pacing in Dog. In Bonagura JD,
Twedt DC (eds): Current Veterinary Therapy XIV. St. Louis, Saunders, 2009, pp
717-721.
Kraus MS, Calvert CA: Syncope. In Bonagura JD, Twedt DC (eds): Current
Veterinary Therapy XIV. St. Louis, Saunders, 2009, pp 709-712.
Aminophylline, Theophylline. In Plumb DC: Plumb's Veterinary Drug Handbook.
Ames, Blackwell Publishing, 2008. 37-40.
Terbutaline Sulfate. In Plumb DC: Plumb's Veterinary Drug Handbook. Ames,
Blackwell Publishing, 2008. 859-860.
12 •consutta