* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Loeffler Endocarditis_ Background, Pathophysiology
Heart failure wikipedia , lookup
Cardiovascular disease wikipedia , lookup
Rheumatic fever wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Coronary artery disease wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
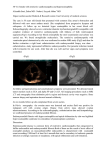
Loeffler Endocarditis: Background, Pathophysiology, Frequency 1z5 http://emedicine.medscape.com/article/155340-overview Loeffler Endocarditis Author: Sohail A Hassan, MD; Chief Editor: Richard A Lange, MD, MBA more... Updated: Feb 17, 2015 Background Loeffler endocarditis and endomyocardial fibrosis are restrictive cardiomyopathies, defined as diseases of the heart muscle that result in impaired ventricular filling with normal or decreased diastolic volume of either or both ventricles. Systolic function and wall thickness may remain normal, especially early in the disease, as reported by Richardson and associates.[1, 2] Both conditions are associated with eosinophilia. The associations among eosinophilia, active carditis, and multiorgan involvement were first described by Loeffler in 1936.[3] Pathologic specimens in Loeffler endocarditis show eosinophilic myocarditis, a tendency toward endomyocardial fibrosis and clinical manifestations of thromboembolism, and acute heart failure. Eosinophilic states that may occur in association with Loeffler endocarditis include hypereosinophilic syndrome, eosinophilic leukemia, carcinoma, lymphoma, drug reactions or parasites, as reported in multiple case series. Although eosinophilic endocardial disease has been well described, myocardial and vascular damage due to eosinophilic infiltration and degranulation is rarely diagnosed during life, as reported by Oakley et al and others.[4] Herzog et al and Tonnesen et al have proposed that the reason for this situation may be the rapidly fatal evolution of most cases of eosinophilic arteritis and myocarditis.[5, 6] These conditions are usually diagnosed based on postmortem examination and nonspecificity of clinical manifestations, as reported by Kim et al, Isaka et al, and Seshadri et al.[7, 8, 9] Pathophysiologically, the fibrotic stage of Loeffler endocarditis is very similar to the disease entity described as endomyocardial fibrosis, which is indolent in comparison to Loeffler endocarditis. The tropical form of endomyocardial fibrosis is associated with eosinophilia, a common finding in Loeffler endocarditis. Pathophysiology Endomyocardial damage in Loeffler endocarditis is well known and described in a study by Solley and associates.[10] Myocardial involvement is less well known and has been considered a manifestation of an acute necrotic stage of eosinophilic endomyocardial disease, as reported by Olsen and colleagues.[11] More recently, cases of isolated eosinophilic myocarditis have been reported without signs of endomyocardial involvement, with or without vasculitis. Additionally, idiopathic eosinophilic endomyocarditis, in the absence of peripheral eosinophilia, has been reported by Priglinger et al.[12] Morphologic abnormalities of eosinophils have been noted in patients with Loeffler endocarditis, suggesting that these eosinophils were mature or stimulated. The intracytoplasmic granular content of activated eosinophils is thought to be responsible for the toxic damage to the heart, as reported by Tai and associates.[13] Spry et al reported eosinophilic degranulation of basic proteins causing myocardial damage in tissue cultures in vitro.[14] Gliech et al reported a dose-dependent cytotoxic effect of the eosinophilic granular proteins, inhibiting multiple enzyme systems.[15] The cationic eosinophilic proteins bind to the anionic endothelial protein, thrombomodulin. This complex impairs anticoagulant activities, leading to enhanced endocardial thrombus formation, as reported by Slungaard and colleagues.[16] Toxins released by the eosinophils include eosinophil-derived neurotoxin, cationic protein, major basic protein, reactive oxygen species, and arachidonic acid derivatives. As described by Cunningham et al, these toxins may cause endothelial and myocyte damage, resulting in thrombosis, fibrosis, and infarction.[17] The intensity and timing of the active carditis is related closely to the severity of the circulating eosinophilia. Some have suggested that, particularly in the tropics, patients who present with later fibrotic stages of endomyocardial disease may have had either transient earlier bouts of moderate eosinophilia with spontaneous resolution, or only moderate levels of eosinophilia leading to a low-grade endomyocarditis with gradual progressive fibrosis, as reported by Olsen et al.[11] Molecular pathophysiology Cools et al reported a landmark finding by treating patients with hypereosinophilic syndrome (HES) with imatinib, a tyrosine kinase inhibitor.[18] Among the findings were the following: The gene defect is localized to an interstitial chromosomal deletion on chromosome band 4q12, resulting in fusion of the Fip1-like1 ( FIP1L1) gene to the platelet-derived growth factor gene alpha ( PDGFRA). The protein product of this gene is a tyrosine kinase enzyme that transforms the hematopoietic stem cells. This FIP1L1-PDGFRA fusion gene defect was identified in 9 of 16 patients treated with imatinib. This study also highlights the importance of reclassifying HES as a myeloproliferative disorder of a possible single clone based on genotyping, as the FIP1L1-PDGFRA gene rearrangement is a clonal abnormality. 8.9.2015 22:29 Loeffler Endocarditis: Background, Pathophysiology, Frequency 2z5 http://emedicine.medscape.com/article/155340-overview Treatment with imatinib caused rapid regression of eosinophilic proliferation and endomyocardiopathy in subsequent cases reported by Vandenberghe et al and Rotoli et al. [19, 20] The following list summarizes the initial clinical presentations of eosinophilic endomyocardial disease in relation to the predominant pathologic stage of the disease as reported by Alderman et al in the Textbook of Cardiovascular Medicine.[21] Death is usually related to multiorgan dysfunction in the presence of congestive heart failure. The initial clinical presentation and stages of eosinophilic endomyocardial disease are descreibed below.[21] Necrotic stage (early stage) Hypereosinophilia with systemic illness (20-30%) is associated with the following signs/symptoms: Fever Sweating Chest pain (as described by Bestetti et al [22] ) Lymphadenopathy Splenomegaly Acute carditis (20-50%) is associated with the following signs/symptoms: Anorexia Weight loss Cough Pulmonary infiltrates Skin and retinal lesion Atrioventricular valve (AV) valve regurgitation Biventricular failure Polymorphic ventricular tachycardia [23] Thrombotic stage Thrombotic emboli (10-20%) are associated with the following signs/symptoms: Cerebral, splenic, renal, and coronary infarction Splinter hemorrhages Fibrotic stage (late stage) Restrictive myopathy (10%) are associated with the following signs/symptoms: AV valvular regurgitation Right and left heart failure The image shows dense fibrosis of ventricle in a postmortem dissected heart. Myocardial as well as valvular involvement with Loffler endocarditis. This image shows dense fibrosis of ventricle in a postmortem dissected heart. Frequency United States The condition is rare and is seen mostly in immigrants from Africa, Asia, and South America. International Loeffler endocarditis is primarily confined to the rain forest (tropical and temperate) belts of Africa, Asia, and South America. Mortality/Morbidity The literature reports a 35-50% 2-year mortality rate in patients with advanced 8.9.2015 22:29 Loeffler Endocarditis: Background, Pathophysiology, Frequency 3z5 http://emedicine.medscape.com/article/155340-overview myocardial fibrosis. Substantially better survival rates may be seen in less symptomatic patients who have milder forms of the disease. As noted, this rate may reflect underdiagnosis of clinically inapparent disease, as for other types of cardiomyopathy. Prognosis The overall prognosis of patients with Loeffler endocarditis is poor and depends on the location of involvement in the heart. The disease is usually slow in onset, with progression to increasing degrees of right and left heart failure. Sudden death and syncope are not as common as in other causes of restrictive cardiomyopathy Race The condition has a predilection for African and African American populations, notably the Rwanda tribe in Uganda, and for people of low socioeconomic status. Whether this is due to genetic factors or the epidemiology of underlying environmental factors is not known. Sex Loeffler endocarditis has a predilection for males. However, endomyocardial fibrosis, which has similar clinical manifestations, is found equally frequently in both sexes. Age The reported age range is 4-70 years. Loeffler endocarditis particularly affects young males, as does its close counterpart, endomyocardial fibrosis, which is more common in children and young adults. Clinical Presentation Contributor Information and Disclosures Author Sohail A Hassan, MD Cardiologist and Cardiac Electrophysiologist, Eastside Cardiovascular Medicine; Director or Electrophysiology at St John Macomb Hospital; Assistant Professor of Medicine, Wayne State University School of Medicine Sohail A Hassan, MD is a member of the following medical societies: American College of Cardiology, American College of Physicians, American Heart Association, Michigan State Medical Society, Heart Rhythm Society Disclosure: Received honoraria from Medtronic, for speaking and teaching; Received honoraria from Zoll for speaking and teaching; Received honoraria from Boeringer Ingelheim for speaking and teaching; Received honoraria from St Jude''''''''s Medical for speaking and teaching; Received honoraria from Boston Scientific corporation for speaking and teaching; Received honoraria from Biotronik for speaking and teaching; Received honoraria from Sanofi Aventis for speaking and teaching. Coauthor(s) Henry Kim, MD, MPH Fellowship Director, Department of Cardiology, Henry Ford Hospital Henry Kim, MD, MPH is a member of the following medical societies: American Medical Association Disclosure: Nothing to disclose. Fatima Ansari University of Michigan Disclosure: Nothing to disclose. Specialty Editor Board Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference Disclosure: Received salary from Medscape for employment. for: Medscape. Marschall S Runge, MD, PhD Charles and Anne Sanders Distinguished Professor of Medicine, Chairman, Department of Medicine, Vice Dean for Clinical Affairs, University of North Carolina at Chapel Hill School of Medicine Marschall S Runge, MD, PhD is a member of the following medical societies: American Physiological Society, American Society for Clinical Investigation, American Society for Investigative Pathology, Association of American Physicians, Texas Medical Association, Southern Society for Clinical Investigation, American Federation for Clinical Research, Association of Professors of Medicine, Association of Professors of Cardiology, American Association for the Advancement of Science, American College of Cardiology, American College of Physicians-American Society of Internal Medicine, American Federation for Medical Research, American Heart Association Disclosure: Received honoraria from Pfizer for speaking and teaching; Received honoraria from Merck for speaking and teaching; Received consulting fee from Orthoclinica Diagnostica for consulting. Chief Editor Richard A Lange, MD, MBA President, Texas Tech University Health Sciences Center, Dean, Paul L Foster School of Medicine Richard A Lange, MD, MBA is a member of the following medical societies: Alpha Omega Alpha, American College of Cardiology, American Heart Association, Association of Subspecialty Professors Disclosure: Nothing to disclose. Acknowledgements Viqar Maria, MD Resident Physician, Department of Internal Medicine, St John Hospital and Medical Center Viqar Maria, MD is a member of the following medical societies: American College of Physicians 8.9.2015 22:29 Loeffler Endocarditis: Background, Pathophysiology, Frequency 4z5 http://emedicine.medscape.com/article/155340-overview Disclosure: Nothing to disclose. References 1. Richardson P, McKenna W, Bristow M, et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation. 1996 Mar 1. 93(5):841-2. [Medline]. 2. Gotlib J. World Health Organization-defined eosinophilic disorders: 2011 update on diagnosis, risk stratification, and management. Am J Hematol. 2011 Aug. 86(8):677-88. [Medline]. 3. Loeffler W. Endocarditis parietalis fibroplastica mit Blut-eosinophilie, ein eigenartiges Krankheitshild. Schweiz Med Wochenschr;. 1936. 66:817-820. 4. Oakley CM, Olsen GJ. Eosinophilia and heart disease. Br Heart J. 1977 Mar. 39(3):233-7. [Medline]. 5. Herzog CA, Snover DC, Staley NA. Acute necrotising eosinophilic myocarditis. Br Heart J. 1984 Sep. 52(3):343-8. [Medline]. 6. Tonnesen P, Teglbjaerg CS. An "unexpected" fatal case of the hypereosinophilic syndrome. Eur J Respir Dis. 1984 Jul. 65(5):389-93. [Medline]. 7. Kim CH, Vlietstra RE, Edwards WD, et al. Steroid-responsive eosinophilic myocarditis: diagnosis by endomyocardial biopsy. Am J Cardiol. 1984 May 15. 53(10):1472-3. [Medline]. 8. Isaka N, Araki S, Shibata M, et al. Reversal of coronary artery occlusions in allergic granulomatosis and angiitis (Churg-Strauss syndrome). Am Heart J. 1994 Sep. 128(3):609-13. [Medline]. 9. Seshadri S, Narula J, Chopra P. Asymptomatic eosinophilic myocarditis: 2 + 2 = 4 or 5!. Int J Cardiol. 1991 Jun. 31(3):348-9. [Medline]. 10. Solley GO, Maldonado JE, Gleich GJ, et al. Endomyocardiopathy with eosinophilia. Mayo Clin Proc. 1976 Nov. 51(11):697-708. [Medline]. 11. Olsen EG, Spry CJ. Relation between eosinophilia and endomyocardial disease. Prog Cardiovasc Dis. 1985 Jan-Feb. 27(4):241-54. [Medline]. 12. Priglinger U, Drach J, Ullrich R, et al. Idiopathic eosinophilic endomyocarditis in the absence of peripheral eosinophilia. Leuk Lymphoma. 2002 Jan. 43(1):215-8. [Medline]. 13. Tai PC, Ackerman SJ, Spry CJ, et al. Deposits of eosinophil granule proteins in cardiac tissues of patients with eosinophilic endomyocardial disease. Lancet. 1987 Mar 21. 1(8534):643-7. [Medline]. 14. Spry CJ, Tai PC, Davies J. The cardiotoxicity of eosinophils. Postgrad Med J. 1983 Mar. 59(689):147-53. [Medline]. 15. Gleich GJ, Frigas E, Loegering DA, et al. Cytotoxic properties of the eosinophil major basic protein. J Immunol. 1979 Dec. 123(6):2925-7. [Medline]. 16. Slungaard A, Vercellotti GM, Tran T, et al. Eosinophil cationic granule proteins impair thrombomodulin function. A potential mechanism for thromboembolism in hypereosinophilic heart disease. J Clin Invest. 1993 Apr. 91(4):1721-30. [Medline]. 17. Cunningham K, Davies RA, Catching J, Veinot JP. Pathologic quiz case: a young woman with eosinophilia and heart failure. Primary hypereosinophilic syndrome with loeffler endocarditis. Arch Pathol Lab Med. 2005 Jan. 129(1):e29-30. [Medline]. 18. Cools J, DeAngelo DJ, Gotlib J, et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003 Mar 27. 348(13):1201-14. [Medline]. 19. Vandenberghe P, Wlodarska I, Michaux L, et al. Clinical and molecular features of FIP1L1-PDFGRA (+) chronic eosinophilic leukemias. Leukemia. 2004 Apr. 18(4):734-42. [Medline]. 20. Rotoli B, Catalano L, Galderisi M, et al. Rapid reversion of Loeffler's endocarditis by imatinib in early stage clonal hypereosinophilic syndrome. Leuk Lymphoma. 2004 Dec. 45(12):2503-7. [Medline]. 21. Alderman EL. Non-infective endocardial disease. Cardiovascular Disease. 1999. 1-7. 22. Bestetti RB, Corbucci HA, Fornitano LD, et al. Angina-like chest pain and syncope as the clinical presentation of left ventricular endomyocardial fibrosis: a case report. Angiology. 2005 May-Jun. 56(3):339-42. [Medline]. 23. Coelho-Filho OR, Mongeon FP, Mitchell RN, Blankstein R, Jerosch-Herold M, Kwong RY. Images in cardiovascular medicine. Loeffler endocarditis presenting with recurrent polymorphic ventricular tachycardia diagnosed by cardiac magnetic resonance imaging. Circulation. 2010 Jul 6. 122(1):96-9. [Medline]. 24. Weller PF, Bubley GJ. The idiopathic hypereosinophilic syndrome. Blood. 1994 May 15. 83(10):2759-79. [Medline]. 25. Maruyoshi H, Nakatani S, Yasumura Y, et al. Loffler's endocarditis associated with unusual ECG change mimicking posterior myocardial infarction. Heart Vessels. 2003 Mar. 18(1):43-6. [Medline]. 26. Mor A, Segev A, Hershkovits R, et al. Hypereosinophilic syndrome presenting as acute myocardial infarction. Allergy. 2000 Sep. 55(9):899-900. [Medline]. 27. Gudmundsson GS, Ohr J, Leya F, et al. An unusual case of recurrent Loffler endomyocarditis of the aortic valve. Arch Pathol Lab Med. 2003 May. 127(5):606-9. [Medline]. 28. Niemeijer ND, van Daele PL, Caliskan K, Oei FB, Loosveld OJ, van der Meer NJ. Loffler endocarditis: a rare cause of acute cardiac failure. J Cardiothorac Surg. 2012 Oct 10. 7:109. [Medline]. [Full Text]. 29. Klag T, Schnetzke U, Benz R, et al. Leriche's syndrome and Loffler endocarditis in a 30-year-old patient presenting with hypereosinophilic syndrome. Ann Hematol. 2012 Jan. 91(1):139-41. [Medline]. 30. Simonnet B, Jacquier A, Salaun E, Hubert S, Habib G. Cardiac involvement in hypereosinophilic syndrome: role of multimodality imaging. Eur Heart J Cardiovasc Imaging. 2014 Oct 9. [Medline]. 31. Parrillo JE. Heart disease and the eosinophil. N Engl J Med. 1990 Nov 29. 323(22):1560-1. [Medline]. 8.9.2015 22:29 Loeffler Endocarditis: Background, Pathophysiology, Frequency 5z5 http://emedicine.medscape.com/article/155340-overview 32. Del Bene MR, Cappelli F, Rega L, Venditti F, Barletta G. Characterization of Loeffler Eosinophilic Myocarditis by Means of Real Time Three-Dimensional Contrast-Enhanced Echocardiography. Echocardiography. 2011 Nov 8. [Medline]. 33. Spyrou N, Foale R. Restrictive cardiomyopathies. Curr Opin Cardiol. 1994 May. 9(3):344-8. [Medline]. 34. Child JS, Perloff JK. The restrictive cardiomyopathies. Cardiol Clin. 1988 May. 6(2):289-316. [Medline]. 35. Watanabe K, Tournilhac O, Camilleri LF. Recurrent thrombosis of prosthetic mitral valve in idiopathic hypereosinophilic syndrome. J Heart Valve Dis. 2002 May. 11(3):447-9. [Medline]. 36. Zakhama L, Slama I, Boussabah E, Harbegue B, Mimouni M, Abdelaali N, et al. Recurrent native and prosthetic mitral valve thrombosis in idiopathic hypereosinophilic syndrome. J Heart Valve Dis. 2014 Mar. 23(2):168-70. [Medline]. 37. Paydar A, Ordovas KG, Reddy GP. Magnetic resonance imaging for endomyocardial fibrosis. Pediatr Cardiol. 2008 Sep. 29(5):1004-5. [Medline]. 38. Langwieser N, von Olshausen G, Rischpler C, Ibrahim T. Confirmation of diagnosis and graduation of inflammatory activity of Loeffler endocarditis by hybrid positron emission tomography/magnetic resonance imaging. Eur Heart J. 2014 Sep 21. 35(36):2496. [Medline]. 39. Arnold M, McGuire L, Lee JC. Loeffler's fibroplastic endocarditis. Pathology. 1988 Jan. 20(1):79-82. [Medline]. 40. Fawzy ME, Ziady G, Halim M, et al. Endomyocardial fibrosis: report of eight cases. J Am Coll Cardiol. 1985 Apr. 5(4):983-8. [Medline]. 41. Felice PV, Sawicki J, Anto J. Endomyocardial disease and eosinophilia. Angiology. 1993 Nov. 44(11):869-74. [Medline]. 42. Uetsuka Y, Kasahara S, Tanaka N, et al. Hemodynamic and scintigraphic improvement after steroid therapy in a case with acute eosinophilic heart disease. Heart Vessels Suppl. 1990. 5:8-12. [Medline]. 43. Butterfield JH, Gleich GJ. Interferon-alpha treatment of six patients with the idiopathic hypereosinophilic syndrome. Ann Intern Med. 1994 Nov 1. 121(9):648-53. [Medline]. 44. Tanaka H, Kawai H, Tatsumi K, Kataoka T, Onishi T, Yokoyama M, et al. Surgical treatment for Loffler's Endocarditis with left ventricular thrombus and severe mitral regurgitation: a case report. J Cardiol. April, 2006. 47 (4):207-13. [Medline]. 45. Jategaonkar S, Butz T, Faber L. [Surgical treatment of the hypereosinophilic syndrome with cardiac involvement (Loffler's endocarditis)]. Dtsch Med Wochenschr. Mar. 2007. 7133(12):50-52. [Medline]. 46. Abonia JP, Putnam PE. Mepolizumab in eosinophilic disorders. Expert Rev Clin Immunol. 2011 Jul. 7(4):411-7. [Medline]. [Full Text]. Medscape Reference © 2011 WebMD, LLC 8.9.2015 22:29