* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download single ventricle indications and evolution
Coronary artery disease wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Heart failure wikipedia , lookup
Myocardial infarction wikipedia , lookup
Aortic stenosis wikipedia , lookup
Cardiac surgery wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Atrial septal defect wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
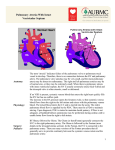
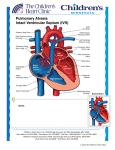
1/23/2009 SINGLE VENTRICLE INDICATIONS AND EVOLUTION Mohammed Al Aklabi 1 1/23/2009 2 1/23/2009 At the end of the talk you should be able to… Identify group(s) of lesions that function physiologically as single ventricles Identify how these babies present to medical attention Know key features of pre-operative stabilization and management Identify the basics and indications of the staged operative approach and postoperative management 3 1/23/2009 3 main ways babies present with congenital heart disease… 1. 2. 3. SHOCK (obstructed flow to body) “BLUE” (obstructed or restricted flow to l lungs) ) HEART FAILURE (excess volume load, ie large AV canal defect) 4 1/23/2009 INTRODUCTION 90% of children with single ventricle lead good quality life with minimal restrictions. Ongoing challenges. Many M unresolved l d controversies. i 5 1/23/2009 EMBRYOLOGY Mechanisms and theories Poor alignment of the common AV valve with Ventricles. Incomplete septation of the ventricular chambers. 6 1/23/2009 7 1/23/2009 Ventricular septation Ventricular septation is a complex process involving different septal structures from various origins and positioned at various planes. 8 1/23/2009 EMBRYOLOGY Formation of the outflow tract and vascular septation. The cardiac outflow tract includes the ventricular outflow tract and the aortopulmonary septum. 9 1/23/2009 ANATOMY Van Praagh’s segmental approach 3 main segments Situs of the patient patient’s s heart: S or I Looping of the ventricles: Dextro or Levo (D or L) Location L ti off the th greatt vessels: l Dextroposition or Levoposition 10 1/23/2009 Anatomy RV morphology Heavily trabeculated Multiple p chordal attachments of the tricuspid valve to the ventricular septal surface LV morphology Smooth finely trabeculated endocardial surface. Tow papillary muscles on the free wall (not p on the septum 11 1/23/2009 Anatomic variants of the single ventricle: NOT just hypoplastic left heart syndrome (HLHS) All generally have mixing of systemic / pulmonary venous return May y also have obstructed venous return 12 1/23/2009 Single ventricle with obstruction to systemic outflow Classic HLHS (small left-sided structures with aortic atresia) Critical aortic stenosis/coarctation Interrupted aortic arch Mitral valve atresia Tricuspid atresia (with transposition of great arteries 13 1/23/2009 Obstruction to pulmonary outflow Tricuspid atresia (normal related great arteries)) Pulmonary atresia Ebstein’s anomaly 14 1/23/2009 Major single ventricle anomalies Tricuspid Atresia including Pulmonary Atresia. ( first successfully managed in 1960s by Fontan) Double inlet single ventricle Unbalanced complete AV canal. Heterotaxy: asplenia/polysplenia syndrome and atrial isomerism. Mitral Atresia including HLHS. 15 1/23/2009 Pathophysiology Parallel versus in series circulation In normal biventricular circulation, blood has pass through g the no choice other than to p systemic resistance. Single ventricle has parallel circulation and flow will depend on both pulmonary and systemic resistances Balanced single ventricle 16 1/23/2009 Clinical Features Dependent on the balance of blood flow between the systemic and pulmonary circulation Spectrum S t off symptoms t based b d on severity it off obstruction Cyanosis, CHF, or shock 17 1/23/2009 Key feature for presentation and management decisions is: Presence of outflow obstruction to lungs OR body? (or both) Is atrial septum open or “restrictive” 18 1/23/2009 The importance of the atrial septum… Single ventricles must have complete mixing at atrial level Restrictive defect will cause high CVP (3rd spacing of fluid…) on systemic side Pulmonary venous hypertension due to high LA pressures can cause significant hypoxemia 19 1/23/2009 Systemic outflow obstruction lesions cont’d… Ductal dependent SYSTEMIC flow Ventricular outflow to lungs predominantly R L shunt at ductal level If no prenatal diagnosis, will present with SHOCK as ductus closes: Poor perfusion, pulses, metabolic acidosis Cyanosis due to profound low systemic output Often accompanying end organ failure (kidney, liver, CNS…) 20 1/23/2009 Keys to adequate resuscitation… RECOGNITION! Ductal dependent lesions just as common as sepsis p in neonatal shock ABC’s--usually involves intubation, sedation Correct metabolic acidosis, usually need fluid boluses +/- inotropy 21 1/23/2009 Resuscitation cont’d… Prostaglandin infusion! Any neonate in shock should be started on PG’s Opens ductus Improves blood flow in ductal-dependent ductal dependent lesions or inter-circulatory mixing in D-TGA Dose 0.01-0.1 mcg/kg/min Side F/X include apnea, vasodilation, fever If worsening i hhypoxia i thi thinkk off pulmonary l venous obstruction 22 1/23/2009 Resuscitation cont’d… Aim for oxygen saturation 70-85% (ie not FiO2 = 100%) Beware of potential for too much pulmonary blood flow (over-circulation) as PVR falls in the first few days Tolerate “highish” PaCO2 to restrict pulmonary blood flow (ductal-dependent systemic circulation) 23 1/23/2009 Diagnostic Workup A thorough clinical assessment. ECG. Ch t x-ray. Chest Oximetry Echocardiogram evaluation. Cardiac cath. MRI 24 1/23/2009 Surgical management Goals : Achieve optimal systemic oxygen delivery in conjunction with low systemic venous pressure. Optimizing compliance of the single ventricle. Optimizing pulmonary arteries growth. Ensure patient will be candidate for Fontan type operation in the future. 25 1/23/2009 Neonatal palliation-stage 1 pulmonary l bl blood d flow: Inadequate: Blalock shunt Central shunt Atrial septectomy TAPVD repair Excessive : PA band SSystemic outflow fl obstruction: Damus kaye stansel Damus-kaye-stansel Norwood procedure 26 1/23/2009 Neonatal palliation-stage 1 Surgery for inadequate pulmonary blood flow Classic Blalock shunt Modified Blalock Central shunt 27 1/23/2009 Surgical stages Stage 1: Norwood type surgery First week of life Stage 2 : Bidirectional cavo-pulmonary connection i ( Gl Glenn shunt h ) 4 – 6 months of age Stage 3 : fenestrated Fontan 1 – 5 years 28 1/23/2009 Norwood operation Classic Norwood operation: Blalock-Taussig shunt 3.5 or o 4 mm graft g a from o innominate or subclavian artery to pulmonary artery Arch augmentation (d ff (different techniques) h ) Atrial septectomy 29 1/23/2009 Modified Norwood operation-Sano Archh augmentation A i Right ventricle to PA conduit (5 mm usually, dependent on size) D Described ib d initially i iti ll b by Norwood but abandoned (large shunts used) Resurrected in Japan by Sano 30 1/23/2009 Sano modification cont’d… Published data: showing less need for “ICU care” to manipulate circulatory balance Seems less prone to sudden circulatory collapse In 2003, malec et al described mortality improvement from 35% with classical Norwood to 5% with Sano 31 1/23/2009 Post-operative management… G General l principles: l Similar to pre-op management of circulation in series with addition of the effects of CPB on PVR/myocardium Emphasis has been on balancing PVR/ / SVR with respiratory or pharmacologic management 32 1/23/2009 Post-operative management… Emphasis on central venous oxygen saturation to estimate adequacy of O2 delivery Remember that difference between arterial and mixed venous saturation is more indicative of overall oxygen delivery 33 1/23/2009 Results 985 neonats between 1994 – 2000 with critical aortic stenosis or atresia, 710 of them underwent stage 1 Norwood procedure. Survival was 76% at one month,, 60% at one year and 54% at five years Risk factors for death included low birth weight, smaller ascending aorta, and older age at the time of the Norwood procedure 34 1/23/2009 Results cont… By 18 months, 58% of patients undergone a bidirectional Glenn shunt Of those who underwent Glenn shunt, 79% y achieved a third - stage g Fontan successfully circulation within 6 years. Mortality for the third stage was 9% 35 1/23/2009 Glenn shunt SVC connection to RPA Cardiac cath. Pre op Measure M pulmonary l resistance 4-6 months of age. 36 1/23/2009 Fontan operation Completion cavopulmonary connection IVC connected to PA L Lateral l tunnell or extracardiac fontan 37 1/23/2009 Fontan operation Extracardiac Fontan With fenestration. 38 1/23/2009 Fontan operation Fontan risk Factors: Pulmonary arterry distortion Pulmonary artery resistance > 2 wood units. AV valve regrgitation Systemic ventricular dysfunction 39 1/23/2009 Is fenestration necessary? Some reports suggested fenestration is not helpful as described by Thompson et all from San Francisco in 1999. Other reports p suggest gg that fenestration is helpful p in reducing morbididty, partiuclarly pleural effusions, shortening hospitalization and possibly reducing mortality 40 1/23/2009 Developmental outcome In 2000 Wernovesky et al reviewed the developmental outcome in 133 patients following Fontan procedure at Children Hospital Boston, mean full scale IQ was 95.7±17.4 which was significantly below normal 41 1/23/2009 summary JJustt lik like allll congenital it l heart h t disease: di needd to t know where the blood goes/flows Counter-intuitive to think of low SpO2 as being better… Rational Under certain morphological circumstances, biventricular repair , although theoretically possible, may not be advisable. 42 1/23/2009 43