* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download At The Heart Of It All
Coronary artery disease wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Remote ischemic conditioning wikipedia , lookup
Antihypertensive drug wikipedia , lookup
Rheumatic fever wikipedia , lookup
Electrocardiography wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Heart failure wikipedia , lookup
Congenital heart defect wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
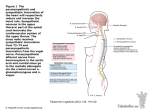
We are seeking participants in a clinical trial to determine the safety and benefits of a new approach to treating heart failure. A t t h e H e a r t o f i t a l l CA U TI O N : Investigational Device. Limited by Federal (or United States) law to investigational use. At the Heart of it all A New Approach to Heart Failure If you have heart failure, you are not alone. It is the fastest growing cardiovascular disorder in the US, and heart failure affects about 23 million people worldwide.1 The brain helps to control the function of the heart through two branches. The sympathetic branch activates the “fight or flight” response during stress, increasing heart rate and blood pressure. The parasympathetic branch has a calming effect on the heart through signals carried from brain to the heart by the vagus nerve. Normally, the two branches are in “balance”. In heart failure, however, there is an imbalance. The sympathetic branch is in overdrive while the parasympathetic branch is underactive. Some heart failure medications help to “slow down” the sympathetic branch, but currently there are no proven treatments that help the underactive parasympathetic branch. Like driving a car, easing up on the accelerator (sympathetic) will slow down the car a bit, but stepping on the brake (parasympathetic) will reduce the speed more quickly. 1 Congestive Heart Failure: Worldwide Drug and Medical Device Market—Kalorama Information available through MarketResearch.com w w w . b i o c o n t r o l - m e d i c a l . c o m 2 At the Heart of it all The CardioFit ® System The CardioFit ® system from BioControl Medical is the first device designed to increase the effect of the parasympathetic branch to help bring better balance to the heart. The system was tested in a European study of people with heart failure.2 Three Main Components of the CardioFit ® System 1. The stimulation lead is a flexible, insulated wire that transmits electrical signals from the stimulator to the vagus nerve. stimulation lead 2. The stimulator is similar to a pacemaker, and contains the electronics of the system in a small sealed case. It is implanted under the skin in the upper chest. 3. A standard pacemaker lead is passed through a vein into a lower chamber of the heart. It is connected to the stimulator, and senses the heartbeat. stimulator standard pacemaker lead 2 De Ferrari GM, Crijns HJ, Borggrefe M, et al. Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur Heart J. 2010; 32(7):847-55 B i o C o n t r o l M e d i c a l 3 The INOVATE-HF Study The INOVATE-HF clinical study will compare the safety and effectiveness of the CardioFit ® system plus optimal medical therapy to optimal medical therapy alone for the treatment of heart failure. The study is seeking up to 650 adult men and women with diagnosed heart failure at selected medical centers in the United States and Europe. The investigators are doctors who specialize in treating heart failure and in managing cardiac devices. Potential candidates are being treated with a combination of medications, but still have symptoms such as shortness of breath and fatigue. i n o v a t e h f @ b i o c o n t r o l - m e d i c a l . c o m 4 Five Steps of the INOVATE-HF Study 1. Evaluation Includes a complete physical examination, questionnaires, and tests to check your overall health status. 2. Randomization For every 5 qualifying participants, 3 will be randomly assigned to receive the CardioFit device and 2 to receive ongoing optimal medical therapy. 3. Implant Trained doctors implant the device for those in the CardioFit group. 4. Adjustment For the participants in the CardioFit group, the therapy is turned on 3 to 5 weeks after implant and is fine-tuned over a 4 week period. Health team members adjust the settings with a computer that communicates with the device through an antenna placed on the chest over the stimulator. 5. Long-term treatment All participants visit their clinic periodically. The visits occur every 3 months for the first 18 months, and every 6 months thereafter until the end of the study (up to 5 1/ 2 years ). i n o v a t e h f @ b i o c o n t r o l - m e d i c a l . c o m 5 About Clinical Research Many new treatments may help people enjoy longer and healthier lives. Clinical studies often compare new treatments with the current standard of care. Strict government regulations control the conduct of clinical studies to protect the rights and ensure the safety of the volunteers. Ideally, the CardioFit ® system will improve heart failure symptoms and slow heart failure progression. While the treatment offered in this study may benefit you, there are no guarantees. We commit to ensuring that INOVATE-HF provides valuable information that will help create better treatments for people with heart failure. Clinical study participation is voluntary. Make sure your doctor answers all your questions and explains the potential risks and benefits. How to Participate in INOVATE-HF If you or someone in your family has heart failure, the INOVATE-HF study may be of interest. Talk to your doctor about the possibility of participating. INOVATE-HF is sponsored by BioControl Medical with funding from strategic partner Medtronic. 9220 Bass Lake Road, Suite 255, New Hope, MN 55428 T: 1-877-494 -8770 | F: 1-763-210 - 5300 inovatehf @ biocontrol-medical.com | www.biocontrol-medical.com © BioControl Medical 2012 6