* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Detection of drug abuse and misuse using biological samples
Orphan drug wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Drug design wikipedia , lookup
Neuropharmacology wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Pharmacognosy wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Urban legends about drugs wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Prescription costs wikipedia , lookup
Drug discovery wikipedia , lookup
Pharmacokinetics wikipedia , lookup
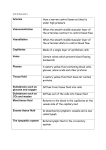
References 1 Melanson, S.E., Baskin, L., Magnani, B., Kwong, T.C., Dizon, A., Wu, A.H. (2010). Interpretation and utility of drug of abuse immunoassays: lessons from laboratory drug testing surveys. Arch Pathol Lab Med 134, 735–739. 2 Becker, M.L., Kallewaard, M., Caspers, P.W., Visser, L.E., Leufkens, H.G., Stricker, B.H. (2007). Hospitalisations and emergency department visits due to drug-drug interactions: a literature review. Pharmacoepidemiol Drug Saf 16, 641–651. 3 D’Onofrio, G., Becker, B., Woolard, R.H. (2006). The impact of alcohol, tobacco, and other drug use and abuse in the emergency department. Emerg Med Clin North Am 24, 925–967. 4 Wu, A.H.B., McKay, C., Broussard, L.A., Hoffman, R.S., Kwong, T.C., Moyer, T.P., Otten, E.M., Welch, S.L., Wax, P. (2003). National Academy of Clinical Biochemistry laboratory medicine practice guidelines: recommendations for the use of laboratory tests to support poisoned patients who present to the emergency department. Clin Chem 39, 357–379. 5 Okie, S. (2010). A flood of opioids, a rising tide of deaths. N Engl J Med 363, 1981–1985. 6 Kuehn, B.M. (2007). Opioid prescriptions soar: increase in legitimate use as well as abuse. JAMA 297, 249–251. 7 US Department of Justice. National drug threat assessment 2011. Available at: www.justice.gov/ndic/pubs44/44849/44849p.pdf [Accessed 1 May, 2012] 8 European Monitoring Centre for Drugs and Drug Addiction. Drug Profiles. Available at: www.emcdda.europa.eu [Accessed 1 May, 2012] 9 Cone, E.J., Huestis, M.A. (2007). Interpretation of oral fluid tests for drugs of abuse. Ann N Y Acad Sci 1098, 51–103. 10 Schepers, R.J., Oyler, J.M., Joseph, R.E. Jr., Cone, E.J., Moolchan, E.T., Huestis, M.A. (2003). Methamphetamine and amphetamine pharmacokinetics in oral fluid and plasma after controlled oral methamphetamine administration to human volunteers. Clin Chem 49, 121–132. 11 Krasowski, M.D., Pizon, A.F., Siam, M.G., Giannoutsos, S., Iyer, M., Ekins, S. (2009). Using molecular similarity to highlight the challenges of routine immunoassay-based drug of abuse/toxicology screening in emergency medicine. BMC Emerg Med 9, 5–22. 12 Crooks, C.R., Brown, S. (2010). Roche DAT immunoassay: sensitivity and specificity testing for amphetamines, cocaine, and opiates in oral fluid. J Anal Toxicol 34, 103–109. 13 Montgomery, E. (2011). Trends in immunoassays for drugs-of-abuse testing. MLO Med Lab Obs 43, 17. 14 Porter, W.H. (2006). Clinical toxicology. In: Tietz textbook of clinical chemistry and molecular diagnostics 4th edition. Burtis, C.A., Ashwood, E.R., Bruns, D.E. eds. St. Louis, MO. Elsevier Saunders, 219–243. 15 Vearrier, D., Curtis, J.A., Greenberg M.I. (2010). Biological testing for drugs of abuse. EXS 100, 489–517. 16 Bosker, W.M., Huestis, M.A. (2009). Oral fluid testing for drugs of abuse. Clin Chem 55, 1910–1931. 17 Drummer, O.H. (2006). Drug testing in oral fluid. Clin Biochem Rev 27, 147–159. COBAS, COBAS C, COBAS E, LIFE NEEDS ANSWERS and ELECSYS are trademarks of Roche. All other trademarks are property of their respective owners. ©2012 Roche Roche Diagnostics International AG CH-6343 Rotkreuz Switzerland www.cobas.com Drugs of abuse testing (DAT) Detection of drug abuse and misuse using biological samples cobas® modular platform Flexible configurations for tailor made solutions Introduction to DAT With the cobas modular platform, the cobas 4000, 6000 analyzer series and cobas 8000 modular analyzer series, Roche has developed a platform concept based on a common architecture that delivers tailor-made solutions for diverse workload and testing requirements. The cobas modular platform is designed to reduce the complexity of laboratory operation and provide efficient and compatible solutions for network cooperation. “More than 1.7 million emergency department visits in 2006 were Flexible and intelligent solutions • Multiple configurations with tailor-made solutions for higher efficiency and productivity • Consolidation of clinical chemistry and immunochemistry with more than 200 parameters for cost and workflow improvements • Future sustainability through easy adaptation to changing throughput and parameter needs cobas 8000 modular analyzer series Large volume <c 502> <e 602> <c 701> cobas 6000 analyzer series Mid volume <c 501> 38 configurations <c 702> 7 configurations <e 601> cobas 4000 analyzer series Low volume <c 311> •C onsistency of interaction with hardware, software and reagents for less training and more staff flexibility • Consistency of patient results due to a universal reagent concept <e 411> 3 configurations associated with drug misuse or abuse, of which, 55 % involved an illicit drug.”1 The use of diagnostic assays for “drugs of abuse” testing (DAT) was originally introduced by criminal justice and law enforcement agencies in order to detect illicit drug use. The scope of testing has since expanded rapidly and led to a huge increase in the number of drug tests performed in both therapeutic and legal/ forensic settings: • Emergency room medicine • Toxicology analysis • Law court-mandated sobriety programs • Penal and residential care rehabilitation programs • Workplace safety programs • Anti-doping monitoring in sport • Crime scene investigations Therapeutic application of DAT aims to improve patient safety and treatment efficacy – a large proportion of patient visits to the emergency department relate to the complications of drug use.2-4 These visits can be the result of drug–drug interactions, intentional or unintentional overdose of prescription or over-thecounter medications, or the use of illicit drugs. Data from the USA show that the mortality rate from unintentional drug overdoses has tripled in the last 15 years.5 For example, the 10-fold increase in prescriptions for opiates observed since 1990 has been accompanied by a 10-fold increase in the number of deaths relating to these drugs.5,6 Therapeutic DAT for a wide range of compounds has been introduced worldwide without significant controversy (see Figure 1 and subsequent section for description of the various classes of drugs of abuse). The use of DAT for the screening of employees in the workplace has, however, proved to be more controversial and large-scale programs are predominantly limited to the USA, where they were first introduced in the public sector during the early 1980s. Private employers were subsequently encouraged to join the “War on Drugs” and private sector screening programs have since become widespread, especially within large companies and corporations. Major drug types identified by DAT In 2007, the estimated cost of illicit drug use to American society totalled more Roche DAT assays than $193 billion, including direct and indirect costs relating to crime, health, Amphetamine • and productivity.7 Barbiturates • Serum Barbiturates • Benzodiazepines • Serum Benzodiazepines • Cocaine • Methadone • Methadone Metabolite (EDDP) • Methaqualone • Opiates • Oxycodone • PCP • PPX • THC • LSD • Specimen Validity (Adulterants) • Psychostimulants • Amphetamine • Methamphetamine • MDMA and derivatives • Cocaine • Ritalin Drugs of abuse testing (DAT) Hallucinogens • Cannabis • Phencyclidine (PCP) • Lysergic acid diethylamide (LSD) Analgesics • Opiates (e.g. heroin, codeine) • Non-opiate opioids (e.g. fentanyl, methadone) Anesthetics (sedatives, hypnotics, narcotics) • Barbiturates (e.g. pentobarbital) • Benzodiazepines (e.g. temazepam) • Ketamine • Alcohol Figure 1: Types of drug commonly investigated by DAT Available Table 2: Roche DAT assay menu (for urine samples unless otherwise indicated) Psychostimulants Amphetamine Amphetamine is a stimulant of the central nervous system (CNS) and increases the pharmacological activity of endogenous neurotransmitters, such as norepinephrine and dopamine. It is occasionally used therapeutically to treat narcolepsy and attention deficit hyperactivity disorder. Because of its stimulating action, the drug has a high potential for abuse and users are at considerable risk of developing tolerance and addiction. Amphetamine is less potent than methamphetamine as it crosses the blood-brain barrier to a lesser extent, but in uncontrolled situations the effects are almost indistinguishable. Amphetamine is usually administered by the oral or intravenous route and is metabolized to predominantly inactive metabolites by oxidative enzymes. The drug appears rapidly in oral fluid following administration and parallels the concentration found in plasma. Excretion of amphetamine via oral fluid and urine is highly dependent on the pH of tissue fluid, which can be influenced by the uptake of acids such as ascorbic acid.8,9 Amphetamine EMIT Enzyme multiplied immunoassay technique Street names Base, speed, whizz RIA Radioimmunoassay Ingestion, insufflation, intravenous injection EIA Enzyme immunoassay Method of administration LC-MS/MS Liquid Chromatography tandem Mass spectrometry Progressive effects HPLC High-performance liquid chromatography GC-MS Gas chromatography-mass spectrometry “Rush” effect; increased confidence, sociability, and energy, fatigue and appetite suppression, restlessness, anxiety, depression, lethargy, amphetamine psychosis Table 1: Methods for detection of drugs Half-life in plasma Detection period in urine (EIA): Oral fluid (GC-MS): 7 – 24 hours Methamphetamine and designer drugs Methamphetamine is similar to amphetamine in terms of pharmacological effects and is the most widely abused, highly addictive synthetic psychotropic drug.8 Methamphetamine can be easily and cheaply manufactured in illicit laboratories and is abused worldwide. The d-isomer is therapeutically used to treat attention deficit hyperactivity disorder and for short-term treatment of obesity; it is considerably more potent than the l-isomer, which is sold over-the-counter as a nasal decongestant. Methamphetamine is metabolized to amphetamine by oxidative enzymes in the liver following administration, but the parent drug remains detectable in oral fluid where its concentration can be four-fold to that which is present in plasma.8–10 In addition to methamphetamine, there are many chemically related “designer drugs” being produced illicitly, including MDMA (‘ecstasy’), MDEA (‘eve’), MDA, MBDB, and PMA/PMMA. These are often produced as tablets that contain a mixture of drugs, including synthetic cannabinoids and/or synthetic cathinones. Most immunoassays for detecting methamphetamine show crossreactivity with MDMA, MDBD, and MDEA. Methamphetamine Street names Crank, crystal meth, ice, meth, pervitin, shabu, speed Method of administration Ingestion, insufflation, intravenous injection, smoked Progressive effects “Rush” effect; increased confidence, sociability and energy, suppression of fatigue and appetite; restlessness, anxiety, depression, lethargy, methamphetamine psychosis Half-life in plasma 8 – 18 hours Detection period in urine (GC-MS): Oral fluid (GC-MS): 22 – 96 hours 6 – 76 hours Occasional use: 24 – 72 hours, Chronic use: 9 days 20 – 50 hours Enzyme immunoassay (EIA) Gas chromatography-mass spectrometry (GC-MS) Table 3: DAT profile of amphetamine9 Gas chromatography-mass spectrometry (GC-MS) Table 4: DAT profile of methamphetamine9 Cocaine Cocaine (chemical name: benzoylmethylecgonine) is a natural compound extracted from the leaves of the coca plant and which has only limited medical use as a topical anesthetic. Purified cocaine has similar biological effects to amphetamine and has been abused as a CNS stimulant since the early 20th century.8 Cocaine is rapidly metabolized and excreted by the kidneys and so only small amounts of the parent drug are usually detectable in urine. Immunoassays that detect cocaine therefore rely on antibodies raised against benzoylecgonine, which is one of the two major cocaine metabolites in humans, and have a low sensitivity for cocaine itself. Commercial immunoassays vary in their detection of other cocaine metabolites, which may lead to differing results if samples are tested using more than one assay system. Cocaine immunoassays generally show a low false-positive rate due to the low structural similarity of benzoylecgonine with common medications or other illicit drugs.11 Cocaine Street names Charlie, coke, crack, snow, white Method of administration Insufflation, intravenous injection, smoked Progressive effects Anesthesia, euphoria, confusion, depression, convulsions, cardiotoxicity Half-life in plasma Detection period in urine (benzoylec gonine, EIA): Oral fluid (benzoylec gonine, GC-MS): Cocaine: 1.4 – 1.8 hours; benzoylecgonine: 5.4 – 7.6 hours Occasional use: 14 – 59 hours; Chronic use: 11 – 147 hours Occasional use: >12 hours; Chronic use: 36 – 72 hours Analgesics Opiates Heroin (chemical name: diacetylmorphine) is a semi-synthetic analgesic opioid used therapeutically for the treatment of severe pain. The illicit form of the drug is usually smoked or solubilized with a weak acid and injected, causing drowsiness, euphoria, and a sense of detachment. Tolerance and physical dependence occur following repeated use and cessation. In tolerant individuals leads to characteristic withdrawal symptoms. Heroin is associated this with far more accidental overdoses and fatal poisonings than any other controlled substance.8 Heroin is difficult to detect in blood samples because it has a plasma half-life of only 3 minutes; it is rapidly hydrolyzed to 6-monoacetylmorphine (6-AM) and then converted to morphine, which is the main active metabolite. 6-AM is a metabolite unique to heroin and can be detected in urine for 24 hours following heroin use. Immunoassays currently marketed for detection of opiate abuse use antibodies raised against morphine. This confers a high sensitivity to structurally related opiates and opioids, such as 6-AM, codeine, hydrocodone, and hydromorphone, but provides little cross-reactivity with oxycodone and its main metabolite oxymorphone. Dedicated oxycodone immunoassays with high sensitivity to oxymorphone have been developed because this drug is a frequently abused prescription medication and is now prescribed in the USA more frequently than codeine, morphine, and propoxyphene. Opiate immunoassays also do not cross-react with synthetic opioids used for opioid maintenance treatment, such as buprenorphine, as well as some commonly used opioid analgesics, such as fentanyl, meperidine (pethidine), and methadone.11 Table 5: DAT profile of cocaine9 Street names Heroin: brown, H, horse, smack; other opiates generally known by local generic or trade names, e.g. codeine, morphine, OxyContin® and Eukodal® (oxycodone), Vicodin® and Lortab® (hydrocodone) Method of administration Ingestion, intramuscular or intravenous injection, smoked Progressive effects Analgesia, sedation, euphoria, nausea, respiratory inhibition, convulsions, coma Half-life in plasma Depends on type of opiate Detection period in urine: Oral fluid (GC-MS): Cannabinoids Street names Block, dope, ganja, grass, green, hash, pot, skunk, weed Method of administration Ingestion, smoked Progressive effects Euphoria; relief of anxiety, sedation, amnesia, disorientation, paranoia Half-life in plasma 5 – 6 days (THC-COOH) Detection period in urine (THC-COOH, EMIT): Oral fluids (THC, RIA): Occasional use: 9 – 78 hours; Chronic use: up to 67 days 2 – 24 hours Occasional use: 7 – 54 hours (opiates, EMIT); Chronic use: up to 11 – 12 days (total morphine, GC-MS) Heroin: 2 – 24 hours, morphine: 2 – 12 hours Enzyme multiplied immunoassay technique (EMIT) Gas chromatography-mass spectrometry (GC-MS) Table 6: DAT profile of opiate drugs9 Phencyclidine Phencyclidine (PCP) was briefly used as a surgical anesthetic but has since been removed from the market due to severe side effects, such as convulsive seizures and hallucinations. Recreational use of PCP peaked in the USA during the 1960s and 1970s and the drug was formally withdrawn in 1978. PCP is usually produced as either a liquid or a powder, but can also be produced in tablet or spray form. Phencyclidine Street names Angel dust, embalming fluid, PCP, rocket fuel Method of administration Ingestion, insufflation, intravenous injection, smoked Progressive effects Dissociative anesthesia, euphoria, altered perception, disorientation, agitation, combativeness, sedation, depression, psychosis, coma Half-life in plasma 7 – 50 hours Detection period in urine (GC-MS): Chronic use: up to 30 days Gas chromatography-mass spectrometry (GC-MS) Table 8: DAT profile of phencyclidine9 Enzyme multiplied immunoassay technique (EMIT) Radioimmunoassay (RIA) Opiates Enzyme immunoassay (EIA) Gas chromatography-mass spectrometry (GC-MS) Hallucinogens Cannabinoids Cannabis (sometimes called marijuana) comprises the dried flowers and leaves of the plant cannabis sativum and is one of the most widely consumed drugs throughout the world. Cannabis is typically smoked, often mixed with tobacco, and long-term use carries the same associated health risks as cigarette smoking. The main psychoactive compound of cannabis is ∆9-tetrahydrocannabinol (THC), which can vary in concentration from 3 – 18 %.8 Screening of urine samples for cannabis is reliable and widely used, although the detection of a positive sample does not provide exact information regarding impairment or the quantity or frequency of use because THC can be deposited in fatty tissue and released into the blood following activity and stress. The inactive metabolite THC-COOH is most prominent in urine whereas THC can be detected in oral fluid because it is deposited in the oral cavity when cannabis is smoked or ingested. Table 7: DAT profile of cannabinoid drugs9 Anesthetics (hypnotics, sedatives, narcotics) Barbiturates Barbiturates are CNS depressants that produce effects ranging from mild sedation to general anesthesia. The parent compound barbituric acid was first synthesized in 1864 and the first pharmacologically active agent, barbital, was introduced in 1904. Only about 50 of the 2,500 synthesized derivatives of barbituric acid have ever been used medically. Due to their narrow therapeutic range, the use of barbiturates as hypnotics/sedatives has largely been superseded by benzodiazepines. Benzodiazepines Benzodiazepines are CNS depressants that induce a feeling of calm and drowsiness, and are more commonly used than barbiturates due to their broader therapeutic range and lower risk of dependence. Benzodiazepines are widely used to treat anxiety, insomnia, and other psychological conditions, and represent a broad family of compounds (clonazepam, diazepam, lorazepam, oxazepam, and many more). Barbiturates can be classified as ultra short-, short-, intermediate-, or long-acting based on the duration of their sedative effect. Ultra short-acting compounds, such as thiopental and methohexital, are used for anesthesia, while long-acting compounds, such as phenobarbital, are used in the treatment of epilepsy and other types of convulsions.8 Benzodiazepine intoxication can be associated with behavioral disinhibition, which can result in hostile or aggressive behavior. The effect is most common when benzodiazepines are taken in combination with alcohol, with combined use also increasing the risk of a fatal overdose because both drugs act as CNS depressants. A similar fatal interaction can occur when benzodiazepines are taken with opiates.8 Barbiturates Benzodiazepines Street names Barbs, downers Street names Benzos, blues, nerve pills Method of administration Ingestion, intramuscular or intravenous injection Method of administration Ingestion, intravenous or intramuscular injection Progressive effects Sedation, lethargy, confusion, respiratory inhibition, coma Progressive effects Drowsiness; dizziness; muscle relaxation Half-life in plasma Depends on type of benzodiazepine; diazepam: 21 – 37 hours Half-life in plasma Detection period in urine (GC-MS): Oral fluid (GC-MS): Depends on type of barbiturate; amobarbital: 20 hours Depends on type of barbiturate; amobarbital: 5 – 6 days Depends on type of barbiturate; amobarbital: 50 hours Detection period in urine (EMIT): Oral fluid (GC-MS): Depends on type of barbiturate; diazepam: 2 – 7 days Depends on type of barbiturate; diazepam: <5 – 50 hours Gas chromatography-mass spectrometry (GC-MS) Enzyme multiplied immunoassay technique (EMIT) Gas chromatography-mass spectrometry (GC-MS) Table 9: DAT profile of barbiturate drugs9 Table 10: DAT profile of benzodiazepine drugs9 New and emerging drugs of abuse Several new types of drug have recently become recognized as drugs of abuse: • Ketamine • Synthetic cannabinoids • Khat and synthetic cathinones • Piperazines and derivatives • Prescription painkillers Ketamine is used as a sedative, analgesic, and anesthetic in emergency medicine, as well as an anti-depressant in some patient groups. The drug can be ingested, insufflated, or injected and the effects are similar to PCP in producing a state of dissociative anesthesia and altered perception that lasts for several hours.8 Synthetic cannabinoids, such as “Spice” in K2 products, are cannabinoid receptor agonists that mimic the effect of THC, although little is known about their detailed pharmacology or toxicology. Synthetic cannabinoids were first developed in the 1980s as investigational drugs by official laboratories, such as the Hebrew University Jerusalem (HU) or Sterling Winthrop (WIN). Some substances are therefore classified according to their place of development, such as HU-210 or WIN-48. Their effects are similar to cannabis but individuals may additionally experience elevated blood pressure and/or paranoia within minutes of use. Fatalities, suicides, and cases of acute psychosis have been reported after consumption of Spice in combination with alcohol and other drugs. High sensitivity LC-MS/MS, GC-MS, and HPLC are currently the state-of-the-art methods used for detection of synthetic cannabinoids, but the large number of clandestine laboratories producing new substances and the lack of reference materials and deuterated internal standards make it difficult to establish standardized testing methods.8 Khat comprises the leaves and fresh shoots of Catha edulis Forsk, which are chewed in order to absorb the two principal active components, cathinone and cathine. Both active components are structurally related to amphetamine and induce similar effects, although they are both less potent. Synthetic cathinones such as mephedrone (also known as “bath salts” or “plant food”) are derivatives of cathinone and are produced illicitly as powders or tablets for ingestion. They are also occasionally found in combination with synthetic cannabinoids in samples of K2 products.8 Analytical methods for DAT “The performance of the Roche DAT assays suggests these new homogeneous screening assays will be an attractive alternative to existing more labor-intensive enzyme immunoassays.”12 Routine DAT is usually a two-step process: 1. Initial batch-wise screening of samples 2. Confirmatory testing of all samples identified as positive Immunoassays were first introduced in the 1970s and are currently the most widely used method for screening biological samples for drugs of abuse.13,14 Immunoassays have become fully automated in order to make DAT as efficient and cost-effective as possible and modern assay platforms are capable of screening hundreds of samples within a short space of time. The disadvantage of high-throughput immunoassays is that they typically only screen for a single compound and can only provide qualitative or semi-quantitative data. Confirmatory testing of samples identified as positive by a screening method is usually required for legal reasons. Reference methods, such as GC-MS and LC-MS/MS, are usually more expensive and technically demanding than immunoassay-based screening. However, results are quantitative and it is possible to identify many different compounds within a single sample. Many emergency departments only have access to confirmatory testing by referral of samples to an off-site reference laboratory and therefore turnaround time is usually not fast enough to aid patient management in real time.11 Biological samples for DAT “While urine remains the most common body fluid used for testing drugs of abuse, over the last several decades the use of alternative matrices, such as blood, sweat, oral fluid, and hair has increased dramatically.”15 Urine is most commonly used for DAT because it is easy to collect in large volumes and does not require pre-analytical preparation. Increasing automation and protocol homogenization have also made urine analysis fast and cost-effective. Blood or serum is also commonly used for DAT, although the taking of a sample requires clinical training and there is a risk of infection even when sterilized equipment is used. The handling and processing of blood and blood products also requires more stringent laboratory protocols compared with other sample types. The main advantages offered by other sample types, such as oral fluid and hair, are the non-invasiveness and simplicity of their collection, which does not require medical staff or special restroom facilities with same-sex collectors. The lack of opportunity for sample adulteration and a lower risk of infection are further advantages. The higher content of parent drug within oral fluid and blood may mean these types of sample are a superior indicator of recent drug use.16 However, the protein content of oral fluid is low compared with blood samples and flow rate can vary depending on the emotional and fed state of the individual.17 Obtaining samples from individuals with dry mouths can be difficult and requires longer collection times. Illicit drugs that reduce oral fluid secretion include amphetamines and cannabis; commonly used drugs having the same effect include antihistamines, antipsychotics, anticholinergics, and some antidepressants. Oral fluid production can be stimulated by the use of agents such as citric acid sweets and chewing gum, but this inevitably changes the pH and concentration of drug in the oral fluid. A range of specialized collection devices has been developed in order to support the collection of oral fluid samples for DAT. These devices are designed to either preserve samples for safe transport to conventional laboratory-based screening or are instead incorporated into a small testing device capable of providing rapid yes/ no determinations. These devices are ideally suited for oral fluid testing used as part of “driving under the influence of drugs” detection programs, criminal justice programs, and the monitoring of medication compliance and workplace safety schemes.16 Diagnostic tests utilizing oral fluid or hair have not been in development as long as urine- or blood-based assays and often rely on non-homogeneous protocols that present challenges to automation and potentially limit laboratory throughput. Perhaps the greatest current limitation of the testing of new sample types, however, is the lack of controlled drug administration studies to inform the interpretation of results. “The use of oral fluid has been found to offer significant promise when detection of relatively recent drug use is sought in a noninvasive manner.”17 The future of DAT The aim of achieving ever more accurate and reliable results using the complementary methods of immunoassay and mass spectrometry, combined with the expanding number of settings in which DAT is being applied, is driving innovation in the field of DAT. Analysis of blood, hair, urine, and oral fluid all present specific advantages and disadvantages depending on the question being asked and it is possible that DAT techniques may diversify further rather than becoming consolidated into a single method suitable for every scenario. Advances in diagnostic and information technology, combined with increasingly stringent legal and medical regulations, should ensure that DAT continues to evolve for the maximum benefit of both patients and healthcare providers. References 1 Melanson, S.E., Baskin, L., Magnani, B., Kwong, T.C., Dizon, A., Wu, A.H. (2010). Interpretation and utility of drug of abuse immunoassays: lessons from laboratory drug testing surveys. Arch Pathol Lab Med 134, 735–739. 2 Becker, M.L., Kallewaard, M., Caspers, P.W., Visser, L.E., Leufkens, H.G., Stricker, B.H. (2007). Hospitalisations and emergency department visits due to drug-drug interactions: a literature review. Pharmacoepidemiol Drug Saf 16, 641–651. 3 D’Onofrio, G., Becker, B., Woolard, R.H. (2006). The impact of alcohol, tobacco, and other drug use and abuse in the emergency department. Emerg Med Clin North Am 24, 925–967. 4 Wu, A.H.B., McKay, C., Broussard, L.A., Hoffman, R.S., Kwong, T.C., Moyer, T.P., Otten, E.M., Welch, S.L., Wax, P. (2003). National Academy of Clinical Biochemistry laboratory medicine practice guidelines: recommendations for the use of laboratory tests to support poisoned patients who present to the emergency department. Clin Chem 39, 357–379. 5 Okie, S. (2010). A flood of opioids, a rising tide of deaths. N Engl J Med 363, 1981–1985. 6 Kuehn, B.M. (2007). Opioid prescriptions soar: increase in legitimate use as well as abuse. JAMA 297, 249–251. 7 US Department of Justice. National drug threat assessment 2011. Available at: www.justice.gov/ndic/pubs44/44849/44849p.pdf [Accessed 1 May, 2012] 8 European Monitoring Centre for Drugs and Drug Addiction. Drug Profiles. Available at: www.emcdda.europa.eu [Accessed 1 May, 2012] 9 Cone, E.J., Huestis, M.A. (2007). Interpretation of oral fluid tests for drugs of abuse. Ann N Y Acad Sci 1098, 51–103. 10 Schepers, R.J., Oyler, J.M., Joseph, R.E. Jr., Cone, E.J., Moolchan, E.T., Huestis, M.A. (2003). Methamphetamine and amphetamine pharmacokinetics in oral fluid and plasma after controlled oral methamphetamine administration to human volunteers. Clin Chem 49, 121–132. 11 Krasowski, M.D., Pizon, A.F., Siam, M.G., Giannoutsos, S., Iyer, M., Ekins, S. (2009). Using molecular similarity to highlight the challenges of routine immunoassay-based drug of abuse/toxicology screening in emergency medicine. BMC Emerg Med 9, 5–22. 12 Crooks, C.R., Brown, S. (2010). Roche DAT immunoassay: sensitivity and specificity testing for amphetamines, cocaine, and opiates in oral fluid. J Anal Toxicol 34, 103–109. 13 Montgomery, E. (2011). Trends in immunoassays for drugs-of-abuse testing. MLO Med Lab Obs 43, 17. 14 Porter, W.H. (2006). Clinical toxicology. In: Tietz textbook of clinical chemistry and molecular diagnostics 4th edition. Burtis, C.A., Ashwood, E.R., Bruns, D.E. eds. St. Louis, MO. Elsevier Saunders, 219–243. 15 Vearrier, D., Curtis, J.A., Greenberg M.I. (2010). Biological testing for drugs of abuse. EXS 100, 489–517. 16 Bosker, W.M., Huestis, M.A. (2009). Oral fluid testing for drugs of abuse. Clin Chem 55, 1910–1931. 17 Drummer, O.H. (2006). Drug testing in oral fluid. Clin Biochem Rev 27, 147–159. COBAS, COBAS C, COBAS E, LIFE NEEDS ANSWERS and ELECSYS are trademarks of Roche. All other trademarks are property of their respective owners. ©2012 Roche Roche Diagnostics International AG CH-6343 Rotkreuz Switzerland www.cobas.com Drugs of abuse testing (DAT) Detection of drug abuse and misuse using biological samples