* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Metal Complexes of N-hydroxyethylnaphthalideneimine Schiff Base
Survey
Document related concepts
Metal carbonyl wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Hydroformylation wikipedia , lookup
Magnetotactic bacteria wikipedia , lookup
Jahn–Teller effect wikipedia , lookup
Coordination complex wikipedia , lookup
Transcript
R. K. MEHTA AND V. C. SINGHI
304
Cyclopentadienyl-tris(
trifluorphosphin)
-mangan
In eine Bestrahlungsapparatur der Firma 0 . Fritz
GmbH, Normschliff-Aufbaugeräte, werden 2,0 g (9,8
mMol) CpMn(CO)3,
gelöst in 340 ml THF, gegeben.
Bei 0 ° C wird mit einem Quecksilberhochdruckbrenner
TQ 150 der Quarzlampen GmbH, Hanau, bestrahlt, wobei sich die roten THF-Komplexe bilden. Danach läßt
man PF3 durch die Lösung perlen, bis sie sich nach
gelb verfärbt hat. Dann bestrahlt man wieder bei 0 ° C
und setzt bei 5 ° C mit PF 3 um. Nach der dritten Bestrahlung muß die Temperatur bei der Einwirkung von
PF 3 auf 20 °C erhöht werden. Dieses Verfahren wiederholt man sechsmal, wobei die Bestrahlungszeit jeweils 8 Stdn. beträgt.
Nach dem Abziehen des Lösungsmittels wird der
schmutziggelbe Rüdestand in Äther aufgenommen und
filtriert. Nach dreimaliger Wiederholung dieser Proze1
29. Mitt.:
T H . K R Ü C K , G . SYLVESTER U. I . P . K U N A U ,
An-
gew. Chem. 8 3 , 725 [ 1 9 7 1 ] ; A n g e w . Chem. internat. Edit.
10, 725 [ 1 9 7 1 ] .
2
T H . K R Ü C K U. L . K N O L L , u n v e r ö f f e n t l i c h t e
3
TH. KRÜCK, Z. Naturforsch. 19 b , 165 [ 1 9 6 4 ] .
4
TH.
KRÜCK, W .
HIEBER U. W .
dur wird bei 40 ° C / 1 0 3 Torr sublimiert. Man erhält
gelbe Kristalle von unangenehmem Geruch.
Ausbeute: 2,6 g = 70% d. Theorie.
Schmp.: 1 6 5 - 1 6 7 ° C .
Analyse:
H
C
F
P
Mn
Ber.:
Gef.:
1,31
1,6
15,64
16,5
44,54
44,5
24,21
23,9
14,13°0
14,2 %
M G W (massenspektr.) : 384.
Unser besonderer Dank gilt dem Ministerium für
Wissenschaft und Forschung des Landes NRW, das
diese Arbeit finanziell unterstützte, und der Badischen
Anilin- & Sodafabrik AG, Ludwigshafen, für die Ausführung der Elementaranalyse.
8
9
208 [ 1 9 6 6 ] ; A n g e w . Chem. internat. Edit. 5, 247
78,
[1966].
11
5
T H . K R Ü C K U. L . K N O L L , i n V o r b e r e i t u n g .
6
TH. KRÜCK U. A. PRASCH, Z . anorg. allgem. Chem. 3 5 6 ,
12
7
M . H E R B E R H O L D U. C . R . JABLONSKI, C h e m . B e r . 1 0 2 ,
118
Ber. 100,
2812
J . M Ü L L E R , K . F E N D E R L U. B . M E R T S C H E K , C h e m . B e r . 1 0 4 ,
700
10
Chem.
S T R O H M E I E R U. F . J . M Ü L L E R , C h e m .
[1967].
Ergebnisse.
LANG, A n g e w .
W.
T.
[1971].
S.
PIPER, F .
A.
COTTON
U. G .
WILKINSON,
J.
inorg.
nuclear Chem. 1 , 1 6 5 [ 1 9 5 5 ] .
Für die A u f n a h m e der Massenspektren danken wir Herrn
D i p l . C h e m . H . VILTER.
[1968].
Für die A u f n a h m e der N M R - S p e k t r e n danken wir Herrn
D r . P . JUNKES.
767
[1969].
Metal Complexes of N-hydroxyethylnaphthalideneimine S c h i f f Base
R . K . M E H T A a n d V . C . SINGHI
Department of Chemistry, University of Jodhpur, J o d h p u r (India)
(Z. Naturforsch. 27 b, received September 14, 1971, revised October 2, 1971)
T h e S c h i f f base, A^-hydroxyethylnaphthalideneimine forms solid complexes with M n ( I I ) ,
Co ( I I ) , N i ( I I ) , Cu ( I I ) , Z n ( I I ) , C d ( I I ) , P d ( I I ) and U 0 2 ( I I ) . T h e structures of these c o m p o u n d s
have been discussed on the basis of their elemental analysis, magnetic moment values and electronic spectral data. These studies have conclusively proved that U 0 2 ( I I ) and M n ( I I ) complexes
are octahedral in shape whereas the C u ( I I ) c o m p l e x molecule displays a square planar or tetragonally distorted octahedral configuration. C o ( I I ) , Ni ( I I ) , Zn (II) and Cd (II) complexes are tetrahedral in structures while the P d (II) c o m p o u n d is square planar.
Although Amaryl and A^-alkyl
Schiff
salicylideneimines
bases and their metal complexes have been
paper describes the results of the investigation on
the complexes of M n ( I I ) , C o ( I I ) , N i ( I I ) , C u ( I I ) ,
intensively studied 1 , those of the ligands in which
Zn(II),
the
droxyethylnaphthalideneimine
amino
groups
are
attached
to
hydroxyalkyl
groups have received little attention 2 ' 3 . It is, there-
Cd(II), Pd(II)
and U 0 2 ( I I )
Schiff
with
base
N-hyand
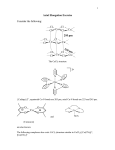
these are represented by the Structures I and II.
fore, considered interesting to study, the metal c o m plexes of the S c h i f f base derived f r o m 2-hydroxy1-napthaldehyde
and
ethanolamine.
The
present
Requests f o r reprints should be sent to Dr. R. K . MEHTA,
Lecturer in Inorganic Chemistry, Department of Chemistry,
Univ. of Jodhpur, Jodhpur ( I n d i e n ) .
Experimental
Materials:
7V-hydroxyethylnaphthalideneimine was
prepared by boiling a mixture of equimolecular proportions of 2-hydroxy-l-naphthaldehyde and ethanolamine
in dry benzene over a waterbath under reflux for two
Dieses Werk wurde im Jahr 2013 vom Verlag Zeitschrift für Naturforschung
in Zusammenarbeit mit der Max-Planck-Gesellschaft zur Förderung der
Wissenschaften e.V. digitalisiert und unter folgender Lizenz veröffentlicht:
Creative Commons Namensnennung-Keine Bearbeitung 3.0 Deutschland
Lizenz.
This work has been digitalized and published in 2013 by Verlag Zeitschrift
für Naturforschung in cooperation with the Max Planck Society for the
Advancement of Science under a Creative Commons Attribution-NoDerivs
3.0 Germany License.
Zum 01.01.2015 ist eine Anpassung der Lizenzbedingungen (Entfall der
Creative Commons Lizenzbedingung „Keine Bearbeitung“) beabsichtigt,
um eine Nachnutzung auch im Rahmen zukünftiger wissenschaftlicher
Nutzungsformen zu ermöglichen.
On 01.01.2015 it is planned to change the License Conditions (the removal
of the Creative Commons License condition “no derivative works”). This is
to allow reuse in the area of future scientific usage.
METAL COMPLEXES OF A-HYDROXYETHYLNAPHTHALIDENEIMINE
hours. Afterwards, excess of benzene was distilled off
and the solid residue was extracted into methanol. The
clear solution was filtered, concentrated and cooled
when yellow crystals of S c h i f f base were obtained.
These were filtered out and recrystallised from methanol, m.p. 145 °C. Found: C 72.41, H 6.02, N 6.49,
[ C 1 0 H 6 ( O H ) C H = N ( C H 2 ) 2 O H ] requires C 72.55, H
6.05, and N 6.51%.
The complexes of M n ( I I ) , Co (II), N i ( I I ) , C u ( I I ) ,
Z n ( I I ) , Cd (II), P d ( I I ) , and U 0 2 ( I I ) with iV-hydroxyethylnaphthalideneimine were prepared by the method
o f Y A M A D A et al.
4
hedral
structure
for
the
305
Mn(II)
complex
which
seems to have been favoured b y steric condition.
Thus
based
on
elemental
analysis,
molecular
weight and magnetic results an octahedral structure
is assigned to this complex in which the third coordination position of the M n ( I I ) is occupied by the
— O H g r o u p and the S c h i f f
base functions as a
tridentate ligand (Fig. 1 ) .
.
All these compounds are found to be insoluble in
water but partially soluble in alcohol, pyridine and
other organic solvents.
Measurements:
The combustion analysis was conducted by using Hosli's electrical micro combustion
furnace. Gallenkamp Semi-micro ebulliometer was
employed for molecular weight determinations using
ethanol as the solvent. The magnetic measurements
were made on Gouy apparatus. Diamagnetic corrections were applied and finally the molar susceptibility
and magnetic moment were evaluated at 303 ° K . The
electronic absorption spectra of the complexes in solution were determined with a VEB Carl Zeiss Jena,
VSU-2P spectrophotometer at room temperature.
Colour, molecular weight and elemental analyses
data of these compounds are given in Table 1 and the
magnetic data in Table 2.
WHERE M=Co(n), Ni(IT), Cu (H),
Z n ( n ) . C d ( n ) , Pd(II) AND U0 2 (H)
WHERE M =Mn (H)
Results and Discussion
Cobalt(II)
The data summarized
Complex:
in
It c o r r e s p o n d s to the
Table 1, give the composition [ C o L 2 ] f o r this c o m -
where L H = [ C 1 0 H 6 ( O H ) C H
p o u n d in which L H = [ C 1 0 H 6 ( O H ) C H = N ( C H 2 ) 2 O H ]
= N ( C H 2 ) 2 O H ] and its magnetic m o m e n t is f o u n d
and thus indicate 1 : 2 metal-ligand stoichiometry in
Manganese(II)
composition
Complex:
[MnL2]
to b e 5 . 8 3 B.M. at r o o m temperature ( 3 0 ° C ) . T h e
it. T h e magnetic moment of this c o m p o u n d at r o o m
magnetic moments of both octahedral o r tetrahedral
temperature ( 3 0 ° C ) is f o u n d to be 4 . 6 1 B.M. The
M n ( I I ) compounds should be nearly 5 . 9 2 B . M . since
electronic
a
of
d i o x a n e and pyridine consist of only one absorption
c o m p l e x e s 5 . The little lower value of the
b a n d with its peak at 1 3 , 7 0 0 c m - 1 . The band may
6S
ground
Mn(II)
state persists in all symmetries
absorption
spectra
of
this c o m p l e x
magnetic moment of this c o m p o u n d m a y b e due to
b e assigned to the transition
spin exchange in its solid state or to the presence of
b e due to the tetrahedral configuration of the c o m -
little M n ( I I )
species which may b e caused due to
aerial oxidation as reported earlier
1
f o r such c o m -
4A2
which may
plex. Thus based on molecular weight spectral and
magnetic data a high-spin tetrahedral structure
(I)
is assigned to the Co ( I I ) complex under study, in
plexes.
In order to decide whether hexacoordination is
due to intermolecular association or to intramolecular combination of
4Tj
in
— O H group with the central
wrhich the S c h i f f
base functions as a bidentate
ligand.
Ni(II)
Complex:
The
greenish
yellow
displaying a metal-ligand ratio of
Ni(II)
M n ( I I ) , the knowledge pertaining to its molecular
complex
weight is quite helpful. The molecular weight of the
can b e represented by the formula [ N i L . , ] , where
1:2,
M n ( I I ) complex under investigation is f o u n d to b e
L H = [ C 1 0 H 6 ( O H ) C H = N ( C H 2 ) o O H ] . The magne-
4 7 5 wrhich excludes the probability of intermolecu-
tic moment of this c o m p o u n d at r o o m temperature
lar association and it is most likely that the intra-
(30 °C)
molecular M — O H b o n d may be present in the c o m -
valent, paramagnetic, high-spin, tetrahedral Ni ( I I )
is f o u n d to b e 3 . 8 2 B.M. In a f o u r co-
pound. This situation is best represented b y an octa-
c o m p l e x the g r o u n d term is 3 T X and the moments lie
R. K. MEHTA AND V. C. SINGHI
306
between
3.2
and 4 . 0 B . M . at r o o m
Based on this the Ni ( I I )
should
display
temperature.
c o m p o u n d under study
tetrahedral
configuration
(I)
in
which the ligand acts as a bidentate one.
ON°
Copper(II)
The molecular weight and
Complex:
the elemental analyses (Table 1) of the olive-green
copper(II)
compound
stoichiometry.
!
given
P C
by
Its
[CuL2]
suggest
1:2
composition
where
may
metal-ligand
therefore
be
L H = [ C 1 0 H 6 ( O H ) CH =
N ( C H 2 ) 2 O H ] . The molecular weight results clearly
C'
indicate its existence as a m o n o m e r in the solid
state. The c o p p e r ( I I ) complex under study exhibits
a magnetic moment of 1.87 B.M. at r o o m temperature ( 3 0 ° C ) . The magnetic-moment of planar-complexes are generally lower
those
'3>3>w ^
<
of
octahedral
(1.8 — 1.9 B . M . )
complexes
(// e ff
than
1.9 — 2 . 0
B . M . ) . As planar stereochemistry may be considered
Tf
r-
C:
K
•O -O
^
O £2
as the limiting case of a tetragonally distorted octa-
rj
hedral stereochemistry, the separation of the interaction terms
=
d.O
( i . e . between ground term
the components of
2To<r
planar
than
which
complexes
possibly
term)
in
explains
2B]?
and
is large in square-
octahedral
the lower
complexes
magnetic
mo-
ments of square-planar complexes 5 ' 6 . The magnetic
moments of perfect tetrahedral complexes are higher
ji
S 43 —1
o
than square planar or octahedral complexes due to
u)£
spin-orbit coupling and are temperature dependent.
o 'S c
T h e electronic absorption spectra in methanol of the
C o p p e r ( I I ) c o m p o u n d consists of a b r o a d band at
about 1 6 7 5 0 c m - 1 and second band at 2 4 2 1 0 c m - 1 .
•. 5
ü
J ®
>5
CH
© TS
©
©
Q PH
o s ?
£—
o
££
T h e appearance of an absorption band at 1 6 7 5 0
« ü c ^
o © —
—
©
O O >>Q
c m - 1 and the magnetic moment value of 1.87 B.M..
support a square planar or tetragonally
octahedral
configuration
of
distorted
the c o m p l e x 7 ' 8 .
The
band exhibit bathochromic shift in pyridine which
may b e attributed to the association of two pyridine
o
E3
O — Cd ON ^ £r3' Ol
o i X. -uo N 3 0
° ° §W
-w
+3
hH Q
S e j e ä S cs K
58 " CS N
: ti C O = O
•SS g •
£ m £ O.S3 T?.S im S "
s
«
• 2 O -S «
.
'S
a >-- © c-i ©
2 0 0 O-S
©®
cK
o
?
;
5
o M
•3 o
K
i o
molecules to the C u ( I I )
ion thus f o r m i n g an octa-
hedral c o m p l e x . The S c h i f f
|o
base appears to ex-
hibit bidentate function in this case.
Wik
-ö
=
K
II - ö Q .
®
-O „ TJ
täZ
II
= .Ü-S O -c ffi
J a
CO
• ^ o i ^ i o
t g x B
X — T? ® >? l O
S O
^-ZO
S®
^ ä «
"7
| II
" — II
oä —
o o i^JL
cS
«
2
©
C 1—1 o
cö ffi
Co
O +J <D
>> s >>
O _
© h- © ^
'- J
OO
Zinc(Il),
CS K
©
x ^ x
C —I c
-ITS ^R-S C
CHO
cS
CO
c
1fl^"H
>,0
,
r ^ : 3 j s . 2 JS ,
3 C O - or^ a ^ 1 ^
CS . 2 -Q •r. jjx ~ cc C . 2 S . 2 l g
t,
5 o £ is M u s i . c a p
§ « 85
dium (II)
o
Cadmium(ll),
Uranyl(II)
and
Palla-
Complexes
These c o m p o u n d s were found diamagnetic, as expected and possess 1 : 2 metal-ligand stoichiometry
as supported by their elemental analysis and molecular weight data. Thus their composition may be
expressed b y [ { C 1 0 H 6 ( O ) C H = N ( C H 2 ) 2 O H } 2 M ( I I ) l ,
where M stands f o r Z n ( I I ) , C d ( I I ) , U 0 2 ( H )
Pd(II).
Consequently
Zn(II)
and
Cd ( I I )
and
com-
METAL COMPLEXES OF A-HYDROXYETHYLNAPHTHALIDENEIMINE
Formulae
[{CI0H6(O)CH = =N(CH2)2OH}2Mn]
[{CI0H6(O)CH= =N(OH2)2OH}2CO]
[{C10H6(O)CH = =N(CH2)2OH}2Ni]
[{CI0H6(O)CH = =N(CH2)20H}2CU]
[{C10H6(O)CH = =N(CH2)2OH}2Zn]
[{CI0H6(O)CH = =N(CH2)2OH }2Cd]
[{CI0H6(O)CH = =N(CH2)20H}2Pd]
[{CI0H6(O)CH = =N(CH2)2OH}2UOo]
Table 2.
Mass Susceptibility
Molar Susceptibility
Magnetic
Moments
No. of
unpaired
7 s x 106
Xm X 10 6
[left in B . M .
electrons
28.2767
17.3498
11.7577
2.4057
13658.34
8447.60
5722.54
1182 4 2
5.83
4.61
3.82
1.87
diamagnetic
diamagnetic
diamagnetic
diamagnetic
M a g n e t i c data of the c o m p l e x e s of iV-hydroxyethylnaphthalideneimine
307
Possible
bond type
sp3d2
sp3
sp3
sp3
Schiff
base at 303 ° K .
pounds possess tetrahedral configuration in which
complex
the S c h i f f
gible difference which indicates that the pyridine
base functions as a bidentate ligand.
in methanol
and pyridine showed
negli-
U 0 2 ( I I ) complex, probably possesses an octahedral
molecules do not coordinate to the central P d ( I I )
structure as is usually displayed b y U 0 2 ( I I )
ion and these results agree with those of YAMADA
this complex the S c h i f f
9.
In
base acts as a bidentate
et a l . 1 0 .
ligand. Pd ( I I ) greatly favours a square planar configuration.
YAMADA et a l . 1 0 h a v e s u g g e s t e d
planar structure
for
a number
of
square
iV-alkylsalicyl-
ideneiminato P d ( I I ) complexes. A similar structure
is suggested f o r the P d ( I I )
complex under investi-
gation, in which the S c h i f f
base functions as a
bidentate ligand. The solution spectra of
1
R . H . H O L M , G . W . E V E R E T T E , JR., a n d A .
Pd(II)
CHAKRAVORTY,
The configurations reported in this communication
S. YAMADA, S. KUGE, a n d K . YAMANOUCHI, B u l l .
Chem.
The authors are thankful to University Grants Commission New Delhi (India) for the award of a fellowship to one of them (V.C.S.).
8
B . N. FIGGIS
[1964].
7
L . SACCONI and M .
S.
N.
PODDAR,
K.
DAY,
J.
HALDAR,
and
NATHSARKAR,
J. Indian Chem. S o c . 4 7 , 743 [ 1 9 7 0 ] .
4
S. YAMADA. H . NISHIKAWA, a n d E . YOSHIDA, B u l l .
B . N . FIGGIS a n d C . M .
1959, 855.
HARRIS. J. c h e m . S o c .
8
9
Chem.
Soc. Japan 39, 9 9 4 [ 1 9 6 6 ] .
5
and
J. LEWIS,
P r o g . Inorg. C h e m . 6, 37
CIAMPOLINI, J . c h e m . S o c .
[London]
1 9 6 4 , 276.
Soc. Japan 40, 1864 [ 1 9 6 7 ] .
3
V-hydroxy-
structurally similar.
Prog. Inorg. Chem. 7, 83 [196612
are in agreement with those of
ethylsalicylideneimine c o m p l e x e s 3 w7ith wrhich it is
[London]
10
L . SACCONI et al., J. Inorg. N u c l . Chem. 1 9 , 73 [ 1 9 6 1 ] .
J. SELBIN, A n g e w . Chem. internat. edn. 5, 7 1 2 [ 1 9 6 6 ] ,
S. YAMADA, Coordin. Chem. Rev. 1, 415 [ 1 9 6 6 ] .