* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download ChemChapter_3[1]
Survey
Document related concepts
Transcript
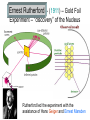
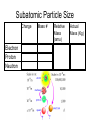
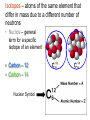
Chapter 3 Atoms: The Building Blocks of Matter Democritus – (460-370 BC) Atom – “indivisible” part of matter Matter can only be divided into smaller parts a limited number of times until you reach the essence of what that matter is. Antoine Lavoisier (1743-1794) – “Father of Modern Chemistry” • Law of the Conservation of Mass – Matter cannot be created or destroyed in a chemical reaction. (1789) Joseph Proust (1754-1826) • The Law of Definite Proportions – a chemical compound contains the same elements in exactly the same proportions regardless of the sample size. • NaCl is always 39.34%Na and 60.66%Cl • H2O is always 11.11%H and 88.89%O John Dalton (1776-1844) • The Law of Multiple Proportions – If two or more compounds are composed of the same two elements, then the ratio of the masses of the second element combined with a certain mass of the first element is always a ratio of small whole numbers. Dalton proposed but couldn’t prove: • Reacting gases and products combine in ratios of small whole numbers. J.L. GayLussac. • Equal volumes of gases, under the same conditions of temperature and pressure, have the same number of molecules. Amedeo Avogadro. Dalton’s Atomic Theory (1808) 1. All matter is composed of small particles called atoms. 2. Atoms of the same element are exactly alike, atoms of different elements are different. 3. Atoms cannot be subdivided, created or destroyed. 4. Atoms of different elements combine in simple whole number ratios to form compounds. 5. In chemical reactions, atoms are simply combined, separated or rearranged. Atomic Structure • Atom – smallest particle of an element that still has the properties of that element. • Nucleus – very small, very dense central portion of the atom where protons and neutrons are located. • Electron Cloud – area surrounding the nucleus where electrons are located. “Discovery” of the Electron • JJ Thomson – (1897) – “found” small negatively charged particles in the beam of a cathode ray tube and called the “corpuscles”. (We call them electrons) He also modified a CRT and found small positive particles (protons) Robert Millikan – (1909) – Oil Drop Experiment • Found the charge to mass ratio of an electron. • From that calculated the mass of an electron. • Me- = 9.109x10-31Kg Ernest Rutherford – (1911) – Gold Foil Experiment – “discovery” of the Nucleus Rutherford led the experiment with the assistance of Hans Geiger and Ernest Marsden Walter Bothe (1930) & James Chadwick (1932) – “discover” Neutron In the 1930s, Bothe found that the radiation emitted by beryllium when it is bombarded with alpha particles was a new form of penetrating high energy radiation, which was later shown by Chadwick to be neutrons. Atomic Model Development Dalton’s Model Thomson’s Plum Pudding Model Quantum Model Bohr’s Model (next chapter) Rutherford’s Model Subatomic Particle Size Charge Electron -1 Proton +1 Neutron 0 Mass # 0 1 1 Relative Actual Mass Mass (Kg) (amu) 0.0005486 9.109x10-31 1.007276 1.673x10-27 1.008665 1.675x10-27 Subatomic Particle Size Charge Electron -1 Proton +1 Neutron 0 Mass # 0 1 1 Relative Actual Mass Mass (Kg) (amu) -31 1836 electrons 9.109x10 0.0005486 equal 1.007276 1 proton 1.673x10-27 1.008665 1.675x10-27 Counting Atomic Particles • Atomic Number (Z) – the number of protons • Mass Number (A) – the total number of protons and neutrons • Number of Neutrons =A-Z Isotopes – atoms of the same element that differ in mass due to a different number of neutrons • Nuclide – general term for a specific isotope of an element • Carbon – 12 • Carbon – 14 Nuclear Symbol ? ? ? Atomic Mass Units – amu- 1/12 the mass of the carbon-12 atom. • Used because atomic masses are so small in Kg and g. • 1 atom of Oxygen-16 = 2.657x10-23g or 15.994915 amu Mole – amount of a substance that contains the same number of particles as 12 g of carbon-12 Avogadro’s Number – the number of particles in one mole of a substance – 6.022x1023 Units can be – atoms/mol or molecules/mol or particles/mol A mole is like a dozen (12) or a gross (144) only it is a mole (6.022x1023) Size of Avogadro ? Molar Mass – mass in grams of one mole of a pure substance. • Numerically equal to the average atomic mass Carbon Iron Atomic Mass (amu) 12.0107 55.845 Molar Mass (g) 12.0107 55.845 Conversions grams – moles - atoms 6.022 x 1023 Atoms of each Example: How many moles are in 22 grams of copper metal? Example: How many atoms are in 22 grams of copper metal? Homework • Pages 89-90 • Numbers 2,4,6,8,11,15,17,18,19,21,22,23