* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download CH 3
Physical organic chemistry wikipedia , lookup
Hydroformylation wikipedia , lookup
Homoaromaticity wikipedia , lookup
Polythiophene wikipedia , lookup
Aromaticity wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Asymmetric induction wikipedia , lookup
Petasis reaction wikipedia , lookup
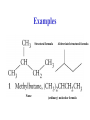
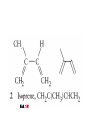
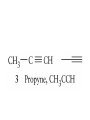
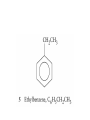
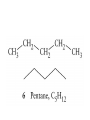
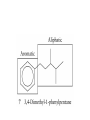
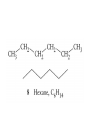
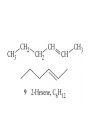
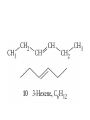
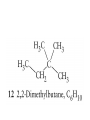
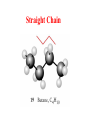
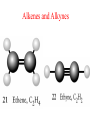
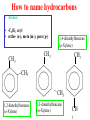
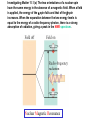
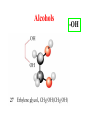
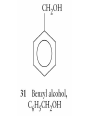
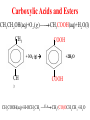
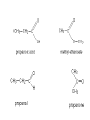
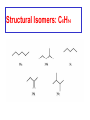
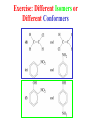
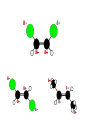
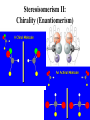
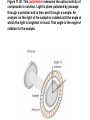
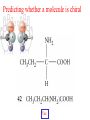
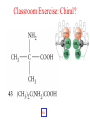
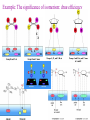
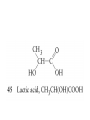
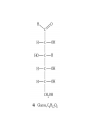
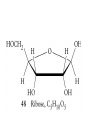
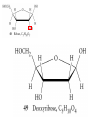
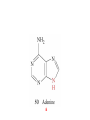
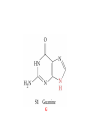
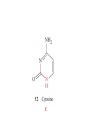
Whenever we cook food, we are acting as organic chemists. The proteins, fats, and carbohydrates that make up our food are organic compounds, and cooking the food causes chemical reactions to occur that decompose some of the compounds into tastier ones. In this chapter we see a little of the amazing variety of organic compounds and study some of their reactions. Assignment for Chapter 11 11.32; 11.40; 11.46; 11.57; 11.67; 11.73; 11.77; 11.78; 11.87;11.92; 11.93 Hydrocarbons and Functional Groups • Alkanes • Alkenes and Alkynes • Aromatic compounds Functional Groups: • Alcohols • Ethers • Phenols • Aldehydes and Ketones • Carboxylic Acids and Esters • Amines and Amides Examples Structural formula Name Abbreviated structural formula (ordinary) molecular formula 異戊二烯 The molecule that shocked science more than once. Friedrich August von Stradonitz Kekulé (1829-1896) who proposed the ring model of benzene in 1861 乙醯柳酸(阿司匹林) 藏茴香酮 (香芹酮) Alkanes: saturated hydrocarbons Cycloalkanes Figure 11.1 The melting and boiling points of the unbranched alkanes from CH4 to C16H34. The dominant intermolecular force in alkanes is the London force because they are nonpolar. Melting point of propane (-187 oC) is lower than that methane (-183 oC) and that of ethane(-172 oC) because of symmetry of methane is higher than that of propane. As the number of carbons increases, the symmetry contribution becomes less and less significant. This initial “glitch” (the anomalous increase of melting point of methane and ethane), therefore, is because of the high symmetry of the two molecules, which provides extra, entropic contribution to enthalpy of melting. Sm H m / Tm (Sm (>0) is smaller for more symmetric molecules , to keep H m constant, Tm must increase for them) Obviously, there is no symmetry contribution to boiling point. Straight Chain Branched (with side chains) Figure 11.2 (a) The atoms in neighboring straight-chain alkanes, represented by the tubelike structures, can lie close together. (b) Fewer of the atoms of neighboring branched alkane molecules can get so close together, so the London forces (represented by double-headed arrows) are weaker and branched alkanes are more volatile. Figure 11.3 The enthalpy changes accompanying the combustion of methane. Although the bonds in the reactants are strong, they are even stronger in the products; and the overall process is exothermic. Figure 11.4 In an alkane substitution reaction, an incoming atom or group of atoms (represented by the orange sphere) replaces a hydrogen atom in the alkane molecule. UV radiation or heat CH4 (g)+Cl2 (g) CH3Cl(g)+HCl(g) Alkenes and Alkynes Figure 11.5 The -bond (yellow electron clouds) in an alkene molecule makes the molecule resistant to twisting around a double bond, so all six atoms lie in the same plane. Figure 11.6 The melting point of an alkene is usually lower than that of the alkane with the same number of carbon atoms. The values shown are for unbranched alkanes and 1-alkenes (that is, alkenes in which the double bond is at the end of the carbon chain). Figure 11.7 In an elimination reaction, two atoms (the orange and purple spheres) attached to neighboring carbon atoms are removed from the molecule, leaving a double bond in their place. Cr2O3 CH3CH3 (g) CH2 =CH2 (g)+H2 (g) Figure 11.8 In an addition reaction, the atoms provided by an incoming molecule are attached to the carbon atoms originally joined by a multiple bond. Addition is the reverse of elimination. CH 2 =CH 2 (g)+Cl2 ( g ) CH 2Cl-CH2Cl Figure 11.9 When bromine dissolved in a solvent (the brown liquid) is mixed with an alkene (the colorless liquid), the bromine atoms add to the molecule at the double bond, a reaction giving a colorless product. CH2 =CH 2 (g)+Br2 (g) CH2Br-CH2Br Aromatic Compounds (Arenes) 萘 蒽 Schematic representation of coal How to name hydrocarbons • Alkanes: • (1) identify the longest unbranched chain and give it the name of the corresponding alkane. • (2)name the alkyl substituent groups by changing the suffix –ane into –yl. Use Greek prefix to indicate how many of each substituents are in the molecule. When different groups are present, list them in alphabetical order and attach them to the root name. • (3) Indicate the locations of the substituents by numbering the backbone carbone C atoms from whichever end of the molecule results in the lower numbers of locations for the substituents. The locations are then written before each substituent, separated by commas. 2,2,4-trimethylpentane 1 1 2 5 2 3 3 4 4 5 Except terminals, wherever there is a C or CH, there is substituent(s). Common mistakes: 2-methyl-2-methyl-4-methylpentane 2-dimethyl-4-methylpentane 4,4,2-trimethylpentane 5 5 1 4 4 33 2 2 1 Using the smallest numbers possible. How to name hydrocarbons • Alkenes and Alkynes: • (1) identify the longest unbranched chain and give it the name of the corresponding alkene or alkyne. • (2)name the alkyl substituent groups by changing the suffix –ane into –yl. Use Greek prefix to indicate how many of each substituents are in the molecule. When different groups are present, list them in alphabetical order and attach them to the root name. • (3) Indicate the locations of the substituents by numbering the backbone carbon C atoms from whichever end of the molecule results in the lower numbers of locations for the substituents. The locations are then written before each substituent, separated by commas. • (4) Number the C atoms in the backbone in the order that gives the lower numbers to the two atoms joined by the multiple bond. The multiple bond has priority over the numbering of substituents. Naming an Alkene 6 5 4 3 2 1 1 2 3 4 5 6 CH3CH=CHCH2CHCH3 CH3 2-methyl-5-hexene 5-methyl-2-hexene The multiple bond has priority over the numbering of substituents 2,3-dimethyl-4-ethylcyclohexene The multiple bond has priority over the numbering of substituents (Use the smallest numbers to locate the double bond) 6 6 5 2 4 3 CH2CH3 5 6 4 3 1 5 1 1 2 2 4 3 CH3 CH3 2,3-dimethyl-4-ethylcyclohexene 6 1 5 4 3 2 6 3 5 CH2 CH3 2 4 4 CH2 CH3 1 2 3 CH3 1 5 6 CH3 CH3 1,6-dimethyl-5-ethylcyclohexene, 5-ethyl-1,6-dimethylcyclohexene, 1,2-dimethyl-3-ethylcyclohexene 4 CH2 CH3 3 5 CH3 6 1 CH3 2 CH3 How to name hydrocarbons • Arenes: • -C6H5 aryl • ortho- (o-), meta (m-), para (p-) 1,4-dimethylbenzene (p-Xylene) CH3 CH3 CH3 CH3 CH3 1,2-dimethylbenzene (o-Xylene) 1,3-dimethylbenzene (m-Xylene) CH 3 Exercise • Name the following hydrocarbons (a) (CH3)2CHCH2CH(CH2CH3)2 1 (b) CH2CH3 2 3 4 5 6 (a) (CH3)2CHCH2CH(CH2CH3)2 (a) 4-ethyl-2-methylhexane (b) 1-ethyl-3-propylbenzene CH2CH2CH3 Quiz • • • • Name and give an example of the major reactions of hydrocarbons. Explain why the melting point and boiling point of a straight-chained hydrocarbon are higher than that a branched hydrocarbon of equal number of carbon atoms. Draw the structures of 4-ethyl-2-methylhexane, 1-ethyl-3-propylbenzene 5-methyl-2-hexene Name the following compounds: CH3 CH3 Functional Groups • • • • • • Alcohols Ethers Phenols Aldehydes and Ketones Carboxylic Acids and Esters Amines and Amides aa Investigating Matter 11.1 (a) The two orientations of a nuclear spin have the same energy in the absence of a magnetic field. When a field is applied, the energy of the spin falls and that of the spin increases. When the separation between the two energy levels is equal to the energy of a radio-frequency photon, there is a strong absorption of radiation, giving a peak in the NMR spectrum. Nuclear Magnetic Resonance Investigating Matter 11.1 (b) The NMR spectrum of ethanol. The red letters denote the protons that give rise to the associated peaks. The NMR spectrum of a molecule is like a fingerprint. Investigating Matter 11.1 (c) An MRI image of a human brain. The patient must lie within the strong magnetic field (background) and the detectors can be rotated around the patient’s head, which allows many different views to be recorded. Magnetic Resonance Imaging makes it possible to see inside a sample noninvasively. Alcohols -OH Ethers R-O-R` Water, CH3CH2-O-H, CH3CH2-O-CH2CH3 Figure 11.12 The boiling points of ethers (given on each column, in degrees Celsius) are lower than those of isomeric alcohols, because hydrogen bonding occurs in alcohols but not in ethers. All the molecules referred to here are unbranched. Phenols 百里香酚 (麝香草酚) 丁香油酚 Aldehydes and Ketones O R-C H O R-C R Smoked meat/fish Wood smoke contains formaldehyde (formalin) that has destructive effect on bacteria so smoked food can be preserved long. Simplest aldehyde: HCHO for aroma of cherries and almonds In oil of cinnamon in oil of vanilla Major Properties • Aldehydes and ketones can be prepared by the oxidation of alcohols. • Aldehydes are reducing agents; ketones are not. Aldehydes:CH3OH(g)+O2 2HCHO(g)+H 2 O(g) 600o C,Ag Na 2 Cr2 O7 (aq),H 2SO4 (aq) Ketones:CH3CH 2 (OH)CH3 CH3CH 2 COCH3 Figure 11.13 An aldehyde (left) produces a silver mirror with Tollens reagent, but a ketone (right) does not. Aldehydes:CH3CH 2CHO+Ag + (from Tollens Reagent) CH3CH2COOH+Ag(s) Ketones:CH3COCH3 +Ag + (from Tollens Reagent) no reaction Carboxylic Acids and Esters CH3CH 2OH(aq)+O2 ( g ) CH3COOH(aq)+H 2 O(l) CH3 COOH + 3O2 (g) +2H2O CH COOH 3 H+ , CH3COOH(aq)+H-OCH2CH3 CH3 (CO)OCH2CH3 +H2O 三硬脂精 Figure 11.14 In a condensation reaction, two molecules are linked as a result of removing two atoms or groups of atoms (the orange and purple spheres) as a small molecule (typically, water). Carboxylic acid + amine amide + water CH3COOH+NH2CH3CH3CONHCH3+H2O Amines and Amides Amines: derivatives from ammonia by replacing hydrogens with organic groups. Amides: resulted from condensation of amines with carboxylic acids. H H H-N-H CH3-N-H H CH3-N-CH3 CH3 CH3-N-CH3 Methylamine Dimethylamine Trimethylamine Carboxylic acid + amine amide + water CH3COOH+NH2CH3CH3CONHCH3+H2O Naming Compounds with Functional Groups • • • • • • • • • • Highlight functional groups. Numbering of carbons should results in lower number for the functional group. Refer to conventions for hydrocarbons Alcohols: alkane-ol CH3CH2CHOHCH32-butanol Ethers: Name each of hydrocarbon groups attached to the O atom separately and alphabetically. CH3OCH2CH3ethyl methyl ether. Aldehydes: identify the parent alkane (including C of –CHO in count of carbon atoms); change the final –e into –al. the –CHO group can occur only at the end of a carbon chain and is given the number 1 only if other substituents need to be located. CH3CH(CHO)CH2CH32methylbutanal. Ketones: Change the –e in parent alkane into –one. the –C=O group is indicated by selecting a numbering order that gives it the lower number. CH3CH2CH2COCH32-pentanone. Carboxylic acids:change the –e of the parent alkane into –oic acid. Include the C atom of the –COOH in count of carbon atoms. CH3CH2CH2COOHbutanoic acid. Esters: Change the –ol of the alcohol to –yl and the oic acid of the parent acid to –oate. CH3CH2COOCH3methyl propanoate. Amines:specify the groups attached to the nitrogen atom in alphabetical order, followed by the suffix –amine. Amines with two amino acids are called diamines. The –NH2 group is called amino- when it is a substituent. (CH3CH2)2NCH3diethylmethylamine. Halides: Name the halogen atom as a substituent by changing the –ine part of a=its name to –o. CH3Brbromomethane. Naming the following compounds (a) CH3CH(CH3)CHOHCH3 (b) CH3CH2CH2COCH3 (c) (CH3CH2)2NCH2CH2CH3 (a) 3-methyl-2-butanol (b) 2-pentanone (c) diethylpropylamine Exercise Naming the following compounds: CH CH OH CH CH (a) CH3CH(CH2CH2OH)CH3 CH (b)CH3CH(CHO)CH2CH3 CH CH CH CH (c) (C6H5)3N 2 3 3 2 3 CHO (a) 3-methyl-1-butanol (b) 2-methylbutanal (c) triphenylamine 3 2 Classroom Exercise Naming the following compounds: (a) CH3CH2CHOHCH2CH3 (b) CH3CH2COCH2CH3 (c) CH3CH2NHCH3 (a) 3-pentanol (b) 3-pentanone (c) ethylmethylamine Quiz 1. Name the following compounds: CH2CH3 CH3CH CHO CH3CH2CH2COOH CH2CH2OH CH3CH CH3 CH3CH2NHCH3 CH3CH2COOCH3 2. What is most important difference between aldelhyde and ketone? 3. Name the following reaction: CH3COOH+NH2CH3CH3CONHCH3+H2O Answer 1. Name the following compounds: CH2CH3 CH3CH CHO 2-methylbutanal CH3CH2CH2COOH butanoic acid CH2CH2OH CH3CH CH3 3-methyl-1-butanol CH3CH2NHCH3 ethylmethylamine CH3CH2COOCH3 methyl propanoate 2. What is most important difference between an aldelhyde and a ketone? An aldelhyde is a good reducing agent, but a ketone is not. 3. Name the following reaction: CH3COOH+NH2CH3CH3CONHCH3+H2O Condensation reaction Isomers • Structural isomers • Stereoisomers: Geometrical isomers Optical isomers Figure 11.15 A summary of the various types of isomerism that occur in molecular compounds. Geometrical and optical isomers are both types of stereoisomers. Structural Isomers: C4H10 CH3-CH2-CH2-CH3 CH(CH3)3 Structural Isomers: C6H14 Isomer vs Conformation They are the same isomer but with different conformations: Exercise: Different Isomers or Different Conformers Stereoisomerism I: Geometric Isomerism Figure 11.16 A pair of geometrical isomers in which two groups are either both on the same side of a double bond (cis) or on opposite sides (trans). Notice that the bonded neighbors of each atom are the same in both cases, but nevertheless the arrangements of the atoms in space are different. cis or trans? (a) trans (b) cis Classroom Exercise: cis or trans? (a) cis (b) trans Figure 11.17 Compounds with rings can also exhibit geometrical isomerism. Groups attached to carbon atoms in a ring can be both on the same face of the ring (cis) or across the plane of the ring from each other (trans). the trans isomer has the higher melting point; the cis isomer has the higher boiling point. 1,2-dichloroethene isomer melting point (°C) boiling point (°C) isomer melting point (°C) boiling point (°C) cis -80 60 cis-but-2-ene -139 4 trans -50 48 trans-but-2-ene -106 1 Why is the boiling point of the cis isomers higher? There must be stronger intermolecular forces between the molecules of the cis isomers than between trans isomers. Why is the melting point of the cis isomers lower? You might have thought that the same argument would lead to a higher melting point for cis isomers as well, but there is another important factor operating. In order for the intermolecular forces to work well, the molecules must be able to pack together efficiently in the solid. Trans isomers pack better than cis isomers. The "U" shape of the cis isomer doesn't pack as well as the straighter shape of the trans isomer. The poorer packing in the cis isomers means that the intermolecular forces aren't as effective as they should be and so less energy is needed to melt the molecule - a lower melting point. Stereoisomerism II: Chirality (Enantiomerism) Figure 11.18 The molecule on the right is the mirror image of the molecule on the left, as can be seen more clearly by inspecting the simplified representations in the circles. Because the two molecules cannot be superimposed, they are distinct optical isomers. All enantiomers have a stereogenic center carbon. This makes the molecule chiral having a non-superimposable mirror image. When we name these enantiomers it is necessary to distinguish them from one another. As it turns out each enantiomer in the pair has opposite configuration. Configuration is the arrangement of the groups attached to a stereogenic center. In one enantiomer the arrangement is clockwise around the stereogenic carbon beginning with the highest priority atom or group. This is called the "R" configuration. The letter "R" comes from the Latin Rectus meaning right. The other enantiomer of the pair being the non-superimposable mirror image will always have an arrangement that proceeds counter clockwise around the stereogenic carbon. This is a different configuration and is called the "S" isomer. The letter "S" comes from the Latin Sinister meaning left. Now if we were to name the two enantiomers using the systematic IUPAC nomenclature system, they would have the same name. We then attach at the beginning of the name the letter "R" or "S" in parenthesis Exercise: Structural isomers? Geometric isomers? Or optical isomers? Conformers Figure 11.19 Plane-polarized light consists of radiation in which all the wave motion lies in one plane (as represented by the orange arrows on the left). When such light passes through a solution of an optically active substance, the plane of the polarization is rotated through a characteristic angle that depends on the concentration of the solution and the length of the path through it. Figure 11.20 This polarimeter measures the optical activity of compounds in solution. Light is plane polarized by passage through a polarizer and is then sent through a sample. An analyzer on the right of the sample is rotated until the angle at which the light is brightest is found. That angle is the angle of rotation for the sample. Predicting whether a molecule is chiral Yes Classroom Exercise: Chiral? CH3 CH3 No CH3 CH3 Example: The significance of isomerism: drug efficiency Quiz • Explain the differences in the melting point and boiling point of trans- and cis- isomers. • Are the following molecules chiral? Polymers (macromolecules) • Synthetic polymers • Biopolymers (DNA, RNA, Carbohydrates, Proteins) Homogenous polymer Heterogeneous polymer Tacticity (stereoregularity) Figure 11.21 The stereoregular polymers produced by using Ziegler-Natta catalysts may be (a) isotactic (all on one side) or (b) syndiotactic (alternating). (c) In an atactic polymer, the substituents lie on random sides of the chain. Case Study 11 This flexible polyacetylene sheet was peeled from the walls of the reaction flask in which it was made from acetylene. Figure 11.22 Collecting latex from a rubber tree in Malaysia, one of its principal producers. Figure 11.23 In natural rubber, the isoprene units are polymerized to be all cis. The harder material, gutta-percha, is the all-trans polymer. Condensation Polymerization: How polymers are synthesized Example: Synthesis of Dacron (Terylene) HOOC COOH + HO-CH2CH2OH O + H2O HOOC C O-CH2CH2OH O HO-CH2CH2OH+ HOOC C O-CH2CH2OH O O C C O-CH2CH2OH HO-CH2CH2O + HOOC COOH O O HOOC C O-CH2CH2O C O C O--CH2CH2O n Figure 11.24 Synthetic fibers are made by extruding liquid polymer from small holes in an industrial version of the spider’s spinneret. Figure 11.25 A scanning electron micrograph of Dacron polyester and cotton fibers in a blended shirt fabric. The cotton fibers have been colored green. Compare the smooth cylinders of the polyester with the irregular surface of cotton. The smooth polyester fibers resist wrinkles, and the irregular cotton fibers produce a more comfortable and absorbent texture. Figure 11.26 A rather crude nylon fiber can be made by dissolving the salt of the amine in water and dissolving the acid in a layer of hexane, which floats on the water. The polymer forms at the interface of the two layers, and a long string can be slowly pulled out. PLAYING AROUND PRODUCES WONDER FIBER--NYLON A team of organic chemists from Du Pont led by Wallace Hume Carothers had been trying to unravel the composition of natural polymers, such as cellulose, silk, and rubber. From this knowledge they hoped to develop synthetic materials that mimicked the properties of these natural polymers. This remarkable group of chemists had developed a group of compounds, polyamides, which had no remarkable or useful properties. These compounds were shelved in order to concentrate their work on a more promising series of compounds, polyesters. Polyesters possessed more desirable properties such as having more soluble products, easier to handle and simpler to work with in the laboratory. Julian Hill, working with polyester, noticed that if you gathered a small amount of this soft polymer on the end of your stirring rod and drew it out of the beaker, it produced a silky, fine fiber. One afternoon when their boss, Wallace Carothers, was not in the lab, the chemists decided to see how long a silky thread they could produce. Hill and his cohorts took a little ball on a stirring rod and ran down the hall and stretched them out into a string. The realization struck them during this horseplay that by stretching the strand of fiber they were orienting the polymer molecules and increasing the strength of the product. The polyesters had very low melting points, too low for textile uses, so they retrieved the polyamides from the shelf and began to experiment with this need 'cold-drawing process.' They found that the strand of polyamide produced by this cold-drawing technique produced a stron g, excellent fiber. The patent for the composition of nylon was never applied for by Du Pont, rather they chose to patent the production process -- cold-drawing -- developed by unsupervised adults playing around in the lab. In January-February 1939, this consumer product hit the US market. It is without equal in its impact before or since. Nylon stockings were exhibited at the Golden Gate International Exposition in San Francisco and were sold first to employees of the inventor company Du Pont de Nemours. On May 15, 1940, nylon stockings went on sale throughout the US, and in New York City alone four million pairs were sold in a matter of hours. Naming this new polymer too many twists and turns. Initially the name norun was proposed for this new product because it was more resistant to laddering than silk. But there were problems and the name was then reversed to read nuron. However, it was pointed out that this was too close to the word neuron which may be construed to be a nerve tonic. Hence, nuron was changed nulon. However this ran into trade mark problems and the name was again changed to nilon. English speakers differed in their pronunciation of this, so, to remove ambiguity the name finally became nylon. Two years before the basic patent on nylon had been filed, the discoverer of nylon, Wallace Hume Carothers, suffering from one of his increasingly frequent attacks of depression, caused by his conviction that he was a scientific failure, drank juice containing potassium cyanide. He would be pleased to know that half of all the chemists in the US work on the preparation, characterization, or application of polymers. Figure 11.27 The strength of nylon fibers is yet another sign of the presence of hydrogen bonds, this time between neighboring polyamide chains. Figure 11.28 The two samples of polyethylene in the test tube were produced by different processes. The floating, lowdensity polymer was produced by high-pressure polymerization. The high-density polymer at the bottom was produced with a Ziegler-Natta catalyst. As the insets show, the higher density results from the greater linearity of the chains, allowing them to pack together better. Figure 11.29 Automobile tires are made of vulcanized rubber and a number of additives, including carbon. The gray cylinders in the small inset represent polyisoprene molecules, and the beaded yellow strings represent disulfide (—S—S—) links that are introduced when the rubber is vulcanized, that is, heated with sulfur. These cross-links increase the resilience of the treated rubber and make it more useful than natural rubber. Figure 11.30 This high-performance race car is made of a composite material that is stronger than steel and can withstand great stress. Biopolymers • Proteins (polypeptides/polyamino acids) • Carbohydrates (polysaccharides) • DNA and RNA (Polynucleotides) They are all heterogeneous polymers: DNA/RNA are four-letter sequences Proteins are 20-letter sequences Carbohydrates are many-letter sequences Proteins • Polymers formed by 20 different residues of amino acids. R side chain C NH3 amino group H COO carboxyl group G A F V L I S T K R H W Y D E C M N Q P Non-polar Amino Acids • There are 8 non-polar amino acids: O H2N CHC OH CH 3 Alanine (A) O H2N CHC OH CH 2 CH 2 S CH 3 Methionine (M) O H2N CHC OH CH CH 3 CH 3 Valine (V) O H2N CHC OH CH 2 O H2N CHC OH CH 2 CHCH 3 CH 3 Leucine (L) O H2N CHC OH CH CH 2 CH 2 CH 2 Isoleucine (I) O C OH HN Proline (P) O H2N CH C OH CH 2 3D structures NH Phenylalanine (F) Trptophan (W) Polar, Uncharged Amino Acids • There 7 polar, uncharged amino acids: O H2N CHC OH H Glycine (G) O H2N CHC OH CH 2 CH 2 C O NH 2 O H2N CHC OH CH 2 OH Serine (S) O H2N CHC OH CH 2 O H2N CHC OH CHOH CH 3 O H2N CHC OH CH 2 SH Threonine (T) Cysteine (C) O H2N CHC OH CH 2 C O NH 2 OH Glutamine (Q) Tyrosine (Y) Asparagine (N) 3D structures Polar, Charged Amino Acids • There are 5 polar charged amino acids: O H2N CHC OH CH 2 N NH Histidine (H) O H2N CHC OH CH 2 CH 2 CH 2 CH 2 NH 2 Lysine (K) O H2N CHC OH CH 2 CH 2 C O OH O H2N CHC OH CH 2 C O OH Glutamic Acid (E) Aspartic Acid (D) O H2N CHC OH CH 2 CH 2 CH 2 NH C NH NH 2 Arginine (R) 3D structures Amino Acids not found in Proteins • Certain amino acids and their derivatives are biochemically important. For example, the visible symptoms of allergies are caused by the release of histamine in mast cells, a type of cell found in loose connective tissue. Histamine dilates blood vessels, increases the permeability of capillaries (allowing antibodies to pass from the capillaries to surrounding tissue), and constricts bronchial air passages. The molecular mechanism of histamine function is by its specific binding to a protein called histamine H1 receptor. H2N CH2 CH2 N Histamine HO NH • H2N CH2 CH2 Serotonin NH Serotonin, which is derived from tryptophan, function as neurotransmitters and regulators. Optical Activity and Stereochemistry of Amino Acids • All amino Acids but glycine are chiral molecules. There are two possible configurations around C that constitute two non-superimposable mirror image isomers, or enantiomers. Enantiomers display optical activity in rotating the plane of polarized light. All natural amino acids are L- isomers. 1 CHO OH OH H CH 2OH H OHC 2 CH 2OH 3 L-Glyceraldehyde (S)-Glyceraldehyde 1 CHO H OH H OH HOH 2C CH 2OH 3 H CH 2OH D-Glyceraldehyde (R)-Glyceraldehyde 2 COOH COOH NH 3 CHO 2 H HOH 2C 3 NH 3 1 L-Serine (S)-Serine Structure of peptide bond • Two amino acids are joined by the peptide bond, a reaction catalyzed by the enzyme called ribosome in all cells: H O H2N C C OH R + NH 3 H O C C OH R' H O H2N C C N R H H O C C OH R' • Due to the double bond character, the six atoms of the peptide bond group are always planar. H C H N C O C C H N C OH C C N C O C + H2O The Level of Protein Structure • Primary Structrue (1º) refers to the amino acid sequences of proteins; • Secondary Structure (2º) refers to segments that constitute structural conformities, or regular structures in proteins; • Tertiary Structure (3º) refers to the folding of protein chains into a more compact three dimensional shape; • Quaternary Structure (4º) refers to organization of subunits (one subunit is a single polypeptide chain). Figure 11.31 A representation of part of an a helix, one of the secondary structures adopted by polypeptide chains. The tubes represent the atoms and their bonds, with colors that correspond to the colors commonly used to represent different atoms. The narrow lines indicate hydrogen bonds. The methyl group side chains show that this molecule is polyalanine. Example: LSPADKTNVK… …VKGWAA… …STVLTSKLYR Figure 11.32 One of the four polypeptide chains that make up the human hemoglobin molecule. Each chain consists of alternating regions of helix (represented by red ribbons) and -pleated sheet. The oxygen molecules we inhale attach to the iron atom (blue sphere) and are carried through the bloodstream to be released where they are needed. Restriction by Amide Plane • Atoms in the peptide bond lie in a plane. Resonance stabilization energy of this planar structure is approximately 88 kJ/mol; • Rotation can only occur around the two bonds connected to the C atom; • Rotation around the Ca and carbonyl bond is called y (psi); • Rotation around the Ca and nitrogen bond is called f (phi). Rotation of Amide Planes • If (f,y) are known for all residues, the structure for the entire backbone is known. • Some (f,y) are more likely than others in a folded protein • Positive (f,y) values correspond to clockwise rotation around bonds when viewed from the C. Zero is defined when the C=O or N-H bond bisects the R-C-H angle. • • • (f,y)=(0,180), two carbonyl oxygens are too close; (f,y)=(180,0), two amide groups are overlapping; (f,y)=(0,0), carbonyl oxygen overlaps with amide group; Classes of Secondary Structures • Terms below define all classes of secondary structures seen in proteins: • Helix -helix – 310 helix • Beta Sheet – Parallel – Anti-parallel • Beta-bulge • Beta Turn The Alpha Helix • • The alpha helix is a helical structure. All alpha helices in proteins are righthanded; H-bond patterns of the alpha helix: – Alpha helix: Carbonyl oxygen of the ith residue forms H-bond with amide proton of the (i+4)th residue. So there are n-4 H-bonds in a helix of n amino acids; – 310 helix: carbonyl oxygen of the ith residue forms H-bond with amide proton of the (i+3)th residue. 3 residues (or 10 atoms) per turn; – Proline is not found in -helix except at the beginning of an -helix; – Helix propensity of an amino acid is a measure of the likelyhood for the amino acid to be in a helix; Glu, Met, Ala, Leu have high propensities; – Examples of -helical proteins include -keratin (structural proteins) and collagen (fibrous protein); – Linus Pauling (Nobel Prize in Chemistry, 1954) figured out the structure of -keratin helix. The Alpha Helix •Residues per turn: 3.6 •Rise per residue: 1.5 Å •Rise per turn: 5.4 Å •(f,y)=~(-60º,-45º) •C=O N-H side chain •Total dipole moment Showing dipole moments The Beta Strands • Beta strands form beta sheet in proteins; • H-bond patterns in beta strands: – Parallel beta-strands (0.325 nm between two residues) – Anti-parallel beta-strands (0.347 nm between two residues) C H R3 O H N H R2 O C R3 R0 O H H N R0 O N O H R2 O H R0 H O R1 H O R3 N N O R1 N N N R2 O N N N H R1 N N H O H N O H O N H R3 R1 N N O C N R0 H N N O H R2 C O The Beta Sheets • • • Formed by beta strands. Note that side chains point away from the sheet while main chains lie on the sheet. Sheets are the most extended form. Sheets consist of parallel strands are usually larger that those consist of anti-parallel strands. A sheet consists of parallel strands distribute hydrophobic residues on both sides of the sheet while that consist of antiparallel strands distributes hydrophobic residues on one side. The Beta Turn (tight turn, or -bend) • Beta turns connect beta strands and reverse the direction of beta strands; • Proline and glycine have high propensity for beta turns; • The carbonyl oxygen of the ith residue forms Hbond with the amide proton of the (i+3)th residue; • Tight turn promotes formation of antiparallel beta sheets. The Beta Bulge • • Beta bulge occurs between normal -strands. Comprised of two residues on one strand and one on the other; Bulges cause bending of otherwise straight anti-parallel beta strands; C C H R3 O H N H N O H R2 O H O R1 H N H R3 N O H R-1 R2 H H N Beta bulge C R1 O N N O H R2 O H R0 H O R1 H O R3 N R3 O N N N O O N N N R0 R1 O H N N N N R0 N R0 H O N N O H R2 O C Anti-parallel strands Super secondary Structures (I) 1 • Hairpins connect two antiparallel strands; 2 • Cross-overs connect two parallel beta strands, most common through an -helix (-- topology). All cross-overs are right-handed. That is, when placing C-side strand closer and pointing right, the connecting ahelix or loop is on the top of the sheet; 1 1 2 2 Right-handed Cross-over Left-handed Cross-over Super Secondary Structures (II) • Coiled-coil is a common alpha helix structure found in proteins that participate in protein folding and protein-protein interactions. – (a-b-c-d-e-f-g)n, where a and d are nonpolar that leads to a hydrophobic side • Helix bundles refers to three or more helices packing together; – Knobs into holes packing: In both kinds of helix packings, slight distortion of the individual helices and the inclination of their axes with respect to each other allows the side chains of the nonpolar residues to mesh together Figure 11.33 The sickle-shaped red blood cells that form when a certain glutamic acid residue in hemoglobin (see Fig. 11.32) is replaced by valine. Figure 11.34 The protein made by spiders to produce a web is a form of silk that can be exceptionally strong. Figure 11.35 The thread on these spools is synthetic spider silk, one of the strongest fibers known. It can be used as the thin, tough thread shown here or wound into cables strong enough to support suspension bridges. Carbohydrates • Carbohydrates are the most abundant organic molecules in nature – Photosynthesis energy stored in carbohydrates; – Carbohydrates are the metabolic precursors of all other biomolecules; – Important component of cell structures; – Important function in cell-cell recognition; – Carbohydrate chemistry: • Contains at least one asymmetric carbon center; • Favorable cyclic structures; • Able to form polymers Carbohydrate Nomenclature (I) • Carbohydrate Classes: – Monosaccharides (CH2O)n • Simple sugars, can not be broken down further; – Oligosaccharides • Few simple sugars (2-6). – Polysaccharides • Polymers of monosaccharides 157 Carbohydrate Nomenclature (II) • Monosaccharide (carbon numbers 3-7) – Aldoses • Contain aldrhyde • Name: aldo-#-oses (e.g., aldohexoses) Memorize all aldoses in Figure ? – Ketoses • Contain ketones • Name: keto-#-oses (ketohexoses) 1 CHO 2 H OH 3 H OH 4 H OH 5 6 H OH CH2OH 1 CHO 2 H O 3 H OH 4 H OH 5 6 H OH CH2OH Polysacchrides • Also called glycans; • Starch and glycogen are storage molecules; • Chitin and cellulose are structural molecules; • Cell surface polysaccharides are recognition molecules. Figure 11.36 The amylose molecule, one component of starch, is a polysaccharide. A polymer of glucose, it consists of glucose units linked together to give a structure like this but with a moderate degree of branching. Polysacchrides • Glucose is the monosaccharides of the following polysacchrides with different linkages and banches (1,4), starch (more branch) (1,4), glycogen (less branch) (1,6), dextran (chromatography resins) (1,4), cellulose (cell walls of all plants) (1,4), Chitin similar to cellulose, but C2-OH is replaced by –NHCOCH3 (found in exoskeletons of crustaceans, insects, spiders) Figure 11.37 The amylopectin molecule is another component of starch. It has a more highly branched structure than amylose. Figure 11.38 (a) Cellulose is yet another polysaccharide constructed from glucose units. The linking between the units in cellulose results in long, flat ribbons that can produce a fibrous material through hydrogen bonding. (b) These long tubes of cellulose formed the structural material of an aspen tree. DNA and RNA A G C T U (in RNA) Extension of the DNA chain Figure 11.41 The condensation of nucleotides that leads to the formation of a nucleic acid—a polynucleotide. The lensshaped object is an attached amine. Figure 11.42 The bases in the DNA double helix fit together by virtue of the hydrogen bonds that they can form as shown on the left. Once formed, the AT and GC pairs are almost identical in size and shape. As a result, the turns of the helix shown on the right are regular and consistent. Figure 11.39 A computer graphics image of a short section of a DNA molecule, which consists of two entwined helices. In this illustration, the double helix is also coiled around itself in a shape called a superhelix. Figure 11.40 A DNA molecule is very large, even in bacteria. In this micrograph, a DNA molecule has spilled out through the damaged cell wall of a bacterium. The Code of Life • Three-letter code of DNA Amino acidsProteins • All other molecules • Organism Assignment for Chapter 11 11.32; 11.40; 11.46; 11.57; 11.67; 11.73; 11.77; 11.78; 11.87;11.92; 11.93