* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Practicalities in the Use of Obesity Pharmacologic Agents
Pharmaceutical industry wikipedia , lookup
NK1 receptor antagonist wikipedia , lookup
Cannabinoid receptor antagonist wikipedia , lookup
Neuropharmacology wikipedia , lookup
Pharmacognosy wikipedia , lookup
Psychopharmacology wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Pharmacogenomics wikipedia , lookup
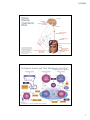
5/15/2015 Practicalities in the Use of Obesity Pharmacologic Agents Caroline M. Apovian, MD, FACN, FACP Professor of Medicine and Pediatrics Boston University School of Medicine Director, Nutrition & Weight Management Center Section of Endocrinology, Diabetes, and Nutrition Boston Medical Center May 14, 2015 2:15 pm-3:15 pm Caroline M. Apovian, MD, FACN, FACP Professor of Medicine and Pediatrics Boston University School of Medicine Director, Nutrition & Weight Management Center Section of Endocrinology, Diabetes, and Nutrition Boston Medical Center Advisory Boards: Research Funding: • Amylin • Lilly • Merck • Amylin • Johnson & Johnson • Arena • Novo Nordisk • Nutrisystem • Zafgen • Sanofi-Aventis • Orexigen • Aspire Bariatrics • GI Dynamics • Pfizer • Sanofi-Aventis • Orexigen • Takeda • MetaProteomics • Dr. Robert C. and Veronica Atkins Foundation • EnteroMedics 1 5/15/2015 U.S. Adult Overweight/Obesity by BMI BMI: <18.5 Underweight U.S. Adult Population 18.5-24.9 25.0-29.9 30.0-34.9 >35 >40 Normal Overweight Obesity I Obesity II Obesity III 31% 34% 20.6% 8.1% 6.4% 35.1% Obese 69% Overweight and Obese More than two-thirds of Americans Ogden CL, Carroll MD, Flegal KM. JAMA. 2014 Jul;312(2):189-90. 8 3 Anti-obesity Drug Treatment Criteria by BMI BMI: <18.5 Underweight U.S. Adult Population 18.5-24.9 25.0-29.9 30.0-34.9 >35 >40 Normal Overweight Obesity I Obesity II Obesity III 31% 34% 20.6% 8.1% BMI >27 kg/m2 with ≥1 comorbidity Ogden CL, Carroll MD, Flegal KM. JAMA. 2014 Jul;312(2):189-90. J Am Coll Cardiol. 2014 Jul 1;63(25 Pt B):2985-3023. Apovian C, et al. J Clin Endocrinol Metab. 2015 Jan 15:jc20143415. [Epub ahead of print] 6.4% BMI >30 kg/m2 with no comorbidities 8 4 2 5/15/2015 Anti-obesity Medications Role in Weight Loss An adjunct to lifestyle modification – not a substitute Can increase chances of meaningful weight loss 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults: A Report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines and The Obesity Society July 1, 2014 J Am Coll Cardiol. 2014 Jul 1;63(25 Pt B):2985-3023. 6 3 5/15/2015 PHARMACOLOGICAL MANAGEMENT of OBESITY: An Endocrine Society Clinical Practice Guideline January 15, 2015 Apovian C, et al. J Clin Endocrinol Metab. 2015 Jan 15:jc20143415. [Epub ahead of print] 7 First Guideline of its Kind: New MD Tool PHARMACOLOGICAL MANAGEMENT of OBESITY: An Endocrine Society Clinical Practice Guideline • Recommended medications and • • • • dosage based on obesity-related comorbidities Specific recommendations for transitioning patients off drugs that cause weight gain Shifts treatment paradigm from treating weight last, to treating weight first Addresses drugs that cause weight gain and alternative drugs that promote weight neutrality or weight loss Lifestyle changes should be a central part of any weight loss medications do not work on their own Bariatric Surgery Adjuncts only Pharmacotherapy Diet Exercise Behavioral Modification Fundamental to all obesity management Roadmap for clinicians considering anti-obesity drug treatment for patients not finding success with diet and exercise alone Apovian C, et al. J Clin Endocrinol Metab. 2015 Jan 15:jc20143415. [Epub ahead of print] 4 5/15/2015 Frequent Patient Follow-up is Key All patients prescribed weight loss medications: JAN FEB MAR APR MAY JUNE JULY At least monthly for first 3 months AUG SEPT OCT NOV DEC Then at least every 3 months Best weight loss outcomes occur with frequent face to face visits 16 visits per year average Centers for Medicare & Medicaid Services Coverage: Month 1 Months 2-6 four visits (1 per week) one visit per month if 3 kg lost, then: Month 7-12 one visit per month www.cms.gov Intensive behavioral therapy for obesity; additional preventive services Watch for new Guidelines on Endocrine Society website at: http://www.endocrine.org/education-and-practice-management/clinical-practice-guidelines 9 Initial Weight Loss Predicts Ultimate Success % reduction in initial weight ILI participants who lost ≥10% at year 1 (N=887) ILI participants who lost 5.0% to 9.9% at year 1 (N=702) N=88 (9.9%) 4 0 N=174 (19.6%) -4 N=177 (25.2%) 4 0 N=247 (35.2%) N=177 (25.2%) -8 -12 N=374 (42.2%) 1 2 3 N=101 (14.4%) -16 4 1 Years 70% had >5% loss at 4 years 0 N=245 (33.6%) N=107 (14.7%) -8 -12 -12 -16 N=325 (44.6%) 4 -4 -4 N=251 (28.3%) -8 ILI participants who lost <5% at year 1 (N=729) 2 3 4 Years 40% had >5% loss at 4 years -16 N=52 (7.1%) 1 2 3 4 Years 22% had >5% loss at 4 years Wadden TA, et al. Obesity. 2011;19(10):1987–1998. 5 5/15/2015 Interactions Among Hormonal and Neural Pathways1 AGRP: agouti‐related peptide; α‐MSH: α‐melanocyte‐stimulating hormone; GHSR: growth hormone secretagogue receptor; INSR: insulin receptor; LepR: leptin receptor; MC4R: melanocortin‐4 receptor; NPY: neuropeptide Y; POMC: proopiomelanocortin; PYY: peptide YY; Y1R; neuropeptide Y1 receptor; Y2R: neuropeptide Y2 receptor. 1. Apovian CM, Aronne, L, Bessesen, D al. J Clin Endocrinol Metab. 2015;100:342‐362. 11 Expected Weight Loss with Approved Anti-obesity Medications Agent Orlistat 60 mg 120 mg Roche, GSK Lorcaserin 10 mg Arena Phentermine plus topiramate-ER (3.75 mg/23 mg for 2 weeks, increased to 7.5 mg/46 mg, escalating to a max of 15 mg/92 mg; 1×)d Vivus Bupropion/ naltrexone Takeda and Orexigen Liraglutide 3.0 mg Novo Nordisk Mechanism of Action Cost Pancreatic lipase inhibitor causing excretion of ~30% of ingested triglycerides in stool 60 mg, $45 120 mg, $207 wholesale Highly selective serotonergic 5-HT2C receptor agonist causing appetite suppression $240 wholesale Noradrenergic + GABA-receptor activator, kainite /AMPA glutamate receptor inhibitor causing appetite suppression $140-195 Inhibitor of dopamine and noradrenaline reuptake + µ opiate antagonist $ 60-70 per month Glucagon-like peptide 1 (GLP-1) agonist 1 Year Weight Loss (kg) 60 mg, −2.5 kg (−1.5 to −3.5) 120 mg, −3.4 kg (−3.2 to −3.6) −3.2 kg (−2.7 to −3.8) 7.5 mg/46 mg −6.7 kg (−5.9 to −7.5) 15 mg/92 mg −8.9 kg (−8.3 to −9.4) 6.2 kg 48 weeks 6.2 kg 104 weeks Common Adverse Effects FDA Approval Oily spotting, flatus with discharge, fecal urgency, fatty oily stool, increased defecation, fecal incontinence 1999 orlistat 2007 OTC Headache, dizziness, fatigue, nausea, dry mouth, cough, and constipation; and in patients with type 2 DM: back pain, cough, and hypoglycemia June 2012 Paresthesias dizziness, taste alterations, insomnia, constipation, dry mouth, elevation in heart rate, memory or cognitive changes July 2012 Nausea, constipation, headache, vomiting, dizziness trouble sleeping, dry mouth diarrhea September 2014 Pancreatitis December 2014 Yanovski SZ and JA.JAMA. 2014;311(1):74-86. Modified from Powell AG, Apovian CM, Aronne LJ. Clin Pharmacol Ther. 2011 Jul;90(1):40-51. 6 5/15/2015 Where Obesity Treatments Work Appetite Suppressing Drugs Hypothalamus VBLOC Device Vagus nerve LAGB surgery Stomach Lipase Inhibitors (Orlistat) Intestines Source of photo: Gastrointestinal peptides controlling body weight homeostasis. Mendieta-Zero H, et al. CIBER of Obesity and Nutrition (ISCIII), Spain; accepted 12 November 2007; available online 21 November 2007. Fat Metabolism Drugs (Beloranib) Adipose Tissue Gastric Bypass, BPD Gastric Sleeve surgeries Intestines Antiobesity Agents and Their Mechanism of Action1 1. Apovian CM et al. J Clin Endocrinol Metab. 2015;100:342-362. 14 7 5/15/2015 Lorcaserin FDA Approved June 27, 2012 Lorcaserin Phase 3 Trials 10 mg QD1 10 mg BID or 10 mg QD2 10 mg BID or 10 mg QD3 Arena Pharmaceuticals 1. Smith SR, et al. N Engl J Med 2010;363:245-56. 2. Fidler MC, et al. J Clin Endocrinol Metab, October 2011, 96(10):3067–3077. 3. O’Neil PM, et al. Obesity (16 March 2012) | doi:10.1038/oby.2012.66 16 8 5/15/2015 Lorcaserin: Weight Loss at Year 1 Smith S, et al. N Engl J Med 2010; 363:245-256. Lorcaserin: Body Weight at Year 1 Smith S, et al. N Engl J Med 2010; 363:245-256. 9 5/15/2015 Lorcaserin: Body Weight at Year 1 and 2 Effects of lorcaserin on body weight during years 1 and 2 among only those patients who continued the study past year 1 Smith S, et al. N Engl J Med 2010; 363:245-256. Lorcaserin: Adverse Events Reported by >5% in Any Group Lorcaserin (N = 3195) Placebo (N = 3185) Headache 537 (16.8) 321 (10.1) Dizziness 270 (8.5) 122 (3.8) Nausea 264 (8.3) 170 (5.3) Constipation 186 (5.8) 125 (3.9) Fatigue 229 (7.2) 114 (3.6) Dry mouth 169 (5.3) 74 (2.3) N (%) Intention-to-Treat Analysis with LOCF Imputation Smith SR, et al. NEJM. 2010;363:245-256. 20 10 5/15/2015 Lorcaserin ─ BLOOM Study: Key Secondary Endpoints Endpoint Lorcaserin Placebo P value Waist circumference (cm) −6.8 −3.9 <0.001 SBP/DBP (mm Hg) −1.4 / −1.1 −0.8 / −0.6 0.04/0.01 Cholesterol (% ∆) Total LDL HDL −0.90 2.87 0.05 0.57 4.03 −0.21 0.001 0.049 0.72 Triglycerides (%) −6.15 −0.14 <0.001 Safety HR (beats/min) Beck depression II −2.0 −1.1 −1.6 −0.9 0.049 0.26 Intention-to-Treat Analysis with LOCF Imputation Smith SR, et al. NEJM. 2010;363:245-256. 21 Phentermine/Topiramate FDA Approved July 2012 11 5/15/2015 Phentermine/Topiramate ER Indications and Dose Mechanism of Action Phentermine • Sympathomimetic amine, NE release Contraindications and Warnings Contraindications • Approved by FDA, July 2012, schedule IV • Indication • Blunts appetite Weight loss in pts with BMI ≥30 kg/m2 or BMI ≥27 kg/m2 with weight-related co-morbid condition(s) Topiramate • Increases GABA activity, antagonize AMPA/ kainate glutamate receptor, carbonic anhydrase inhibitor • Treatment Dose Daily phentermine 7.5 mg topiramate ER 46 mg • Max Dose Daily phentermine 15 mg topiramate ER 92 mg • Prolongs satiety Pregnancy, glaucoma, hyperthyroidism, MAOIs Warnings • Fetal toxicity • Increased heart rate • Suicide and mood and sleep disorders • Acute myopia and glaucoma • Cognitive impairment • Metabolic acidosis • Creatinine elevations • Hypoglycemia with diabetes meds Phentermine and topiramate extended-release [package insert]. Mountain View, CA: Vivus; 2012. 23 Phentermine/Topiramate ER • • Once-a-day, oral, extended-release topiramate Low doses of previously approved medications to minimize side effects 23 46 Maximum Approved Doses 92 Topiramate ER 0 50 10 0 150 200 250 300 350 400 mg Phentermine 0 3.75 Low 5 7.5 Mid 10 15 Full 20 25 30mg (free base) DOSING • Begin with low dose for 2 wks phentermine 3.75/ topiramate ER 23 • Advance to treatment dose phentermine 7.5/ topiramate ER 46 • If <3% weight loss after 12 wks, either discontinue or advance to full dose phentermine 15/ topiramate ER 92 (transition dose phentermine 11.25/ topiramate ER 69 for 2 wks) • If <5% weight loss after 12 wks on full dose, discontinue (take every other day for 1 wk) Phentermine and topiramate extended-release [package insert]. Mountain View, CA: Vivus; 2012. 12 5/15/2015 Percent Weight Loss with PHEN/TPM ER 7.5/46 and Individual Components P<.05 vs. placebo at all time points except week 0 for all comparisons between PHEN/TPM ER and placebo or the individual components except PHEN/TPM ER 7.5/46 vs. topiramate ER 92 at weeks 20, 24, and 28 in the mITT population Aronne LJ, et al. Obesity (Silver Spring). 2013 Nov;21(11):2163-71. 25 Percent Weight Loss with PHEN/TPM ER 7.5/46 and Phentermine 15 and Topiramate P<.05 vs. placebo at all time points except week 0 for all comparisons between PHEN/TPM ER and placebo or the individual components except PHEN/TPM ER 7.5/46 vs. topiramate ER 92 at weeks 20, 24, and 28 in the mITT population Aronne LJ, et al. Obesity (Silver Spring). 2013 Nov;21(11):2163-71. 26 13 5/15/2015 Weight loss at 28 weeks with lifestyle intervention and placebo, phentermine and phentermine/topiramate ER 0 N=103 N=104 N=106 N=103 -1 Weight Loss (%) -2 -1.71 Placebo -3 Phentermine 7.5 mg -4 Phentermine 15 mg -5 -5.45 -6 Phentermine/Topirmate Topiramate ER 7.5/46 mg -6.06 -7 -8 -8.46 -9 Data shown are LS mean and all comparisons are statistically significant. Treatment arms not shown are topiramate 46 mg, topiramate 92 mg and phentermine/topiramate ER 15/92 mg. Aronne LJ, et al. Obesity (Silver Spring). 2013 Nov;21(11):2163-71. 27 Randomized comparison of weight loss at 28 weeks with lifestyle intervention and placebo, phentermine 7.5 mg or phentermine 15 mg Aronne LJ, et al. Obesity 2013. 21, 1-9. 0 N=103 N=104 N=106 N=103 Weight Loss (%) -1 -2 -1.71 Placebo Phentermine 7.5 mg Phentermine 15 mg -3 -4 -5 -6 -5.45 -6.06 -7 Data shown are LS mean and all comparisons are statistically significant. Treatment arms not shown are topiramate 46 mg and 92 mg and phentermine/topiramate ER 7.5/ 46 mg and 15/92 mg. 14 5/15/2015 Effect of Phentermine/Topiramate ER on Weight Loss in Obese Adults Over 2 Years SEQUEL Study Placebo -1.8% Phentermine/topiramate CR 7.5/46 -9.3% -10.5% Phentermine/topiramate CR 15/92 Data are shown with least squares mean (95% CI). Garvey WT, et al. Am J Clin Nutr. 2012;95:297-308. 29 Phentermine/Topiramate ER Improves Risk Factors and Manifestations of Cardiometabolic Disease CONQUER Study Changes from baseline to week 56 in secondary endpoints Phentermine 7.5mg/ Topiramate 46 mg ER Placebo P value Waist circumference (cm) -7.6 -2.4 <0.0001 Systolic BP (mm Hg) -4.7 -2.4 0.0008 -3.4 -2.7 0.1281 -8.6 4.7 <0.0001 -3.7 -4.1 0.7391 Variable Diastolic BP (mm Hg) Triglycerides (%) LDL–C (%) HDL–C (%) 5.2 1.2 <0.0001 CRP (mg/L) -2.49 -0.79 <0.0001 Adiponectin (µg/mL) 1.40 0.33 <0.0001 Gadde KM, et al. Lancet. 2011;377(9774):1341-1352. 30 15 5/15/2015 Phentermine/Topiramate ER: EQUIP and CONQUER Most Commonly Reported Treatment Emergent Adverse Events Placebo PHEN/TPM ER 3.75/23 PHEN/TPM ER 7.5/46 PHEN/TPM ER 15/92 Paresthesia 1.9 4.2 13.7 19.9 Dry mouth 2.8 6.7 13.5 19.1 Constipation 6.1 7.9 15.1 16.1 Upper respiratory tract infection 12.8 15.8 12.2 13.5 Headache 9.3 10.4 7.0 10.6 Dysgeusia 1.1 1.3 7.4 9.4 Nasopharyngitis 8.0 12.5 10.6 9.4 Insomnia 4.7 5.0 5.8 9.4 Dizziness 3.4 2.9 7.2 8.6 Sinusitis 6.3 7.5 6.8 7.8 Nausea 4.4 5.8 3.6 7.2 Back pain 5.1 5.4 5.6 6.6 Fatigue 4.3 5.0 4.4 5.9 Blurred vision 3.5 6.3 4.0 5.4 Diarrhea 4.9 5.0 6.4 5.6 Adverse Event (%) (N=3749) Phentermine and topiramate extended-release [package insert]. Mountain View, CA: Vivus; 2012. 31 Lorcaserin vs. Phentermine/topiramate • Both lorcaserin (Belviq) and the phentermine/topiramate ER combination (Qsymia) taken as adjuncts to diet and exercise may be effective in increasing weight loss in the first year of use, but much less so in the second year • Qsymia appears to be more effective than lorcaserin, but may cause more troublesome adverse effects JAMA September 3, 2014 Volume 312, Number 9. Two Drugs for Weight Loss from: The Medical Letter on Drugs and Therapeutics. September 3, 2012;54(1398):69-71. 16 5/15/2015 Naltrexone/Bupropion FDA Approved September 11, 2014 Naltrexone/Bupropion • Mechanism of Action – Naltrexone ─ Opioid receptor antagonist – Bupropion ─ Dopamine/noradrenaline reuptake inhibitor • Approved by FDA committee but FDA did not approve until a CVD outcome study is performed due to concerns about blood pressure and pulse in some patients • The Light Study (CVD outcomes) is under way; estimated completion: July 2017 Apovian C, et al. Obesity (Silver Spring). 2013 May;21(5):935-43. Clinicaltrials.gov. Cardiovascular Outcomes Study of Naltrexone SR/Bupropion SR in Overweight and Obese Subjects With Cardiovascular Risk Factors (The Light Study). 2012. http://clinicaltrials.gov/show/NCT01601704 34 17 5/15/2015 Naltrexone/Bupropion: Mechanism of Action • Sustained-release combination of the dopamine and norepinephrine reuptake antagonist bupropion and the opioid antagonist naltrexone • Proposed dual mechanism of action involves complementary stimulation of central melanocortin pathways, resulting in increased energy expenditure and reduced appetite Padwal R. Curr Opin Investig Drugs. 2009 Oct;10(10):1117-25. 35 Naltrexone/Bupropion: Dosages NB32 • Sustained-release naltrexone 32 mg/day plus sustained-release bupropion 360 mg/ day combined in fixed-dose tablets NB16 • Sustained-release naltrexone 16 mg/day plus sustained-release bupropion 360 mg/day combined in fixed-dose tablets http://ir.vivus.com/releasedetail.cfm?ReleaseID=407933 36 18 5/15/2015 Naltrexone/Bupropion COR-I Phase 3 Mean Weight Loss 56 Weeks – Completer Population Placebo Naltrexone 16 mg + bupropion Naltrexone 32 mg + bupropion 37 Greenway FL, et al. Lancet 2010 Aug 21; 376:595. DOI:10.1016/S0140-6736(10)60888-4. COR-I and COR-II: Body Weight, Percent Change from Baseline Naltrexone/Bupropion Observed 0 ITT‐LOCF Observed# 0 (N=456) -1.9% -4 -1.3% -5.0%* -6 NB16 -6.8%* (N=471) -6.1%* -8 Change from baseline (%) Change from baseline (%) (N=511) -2 ITT‐LOCF Placebo Placebo -1.4% -1.2% -2 -4 -6 NB32 (N=702) -8 -8.1%* NB32 -8.2%* -10 (N=471) 0 8 16 24 32 40 48 56 56 -10 0 8 16 24 Week COR‐I Completers Placebo (N=290): ‐1.8% NB16 (N=284): ‐6.7%* NB32 (N=296): ‐8.1%* -6.4%* 32 40 48 56 56 Week COR‐II Completers Placebo (N=267): ‐1.4% NB32 (N=434): ‐8.2%* #COR‐II: NB observed data are NB32/NB48 pooled (N=825), no differences were observed for subjects re‐randomized to NB32 vs. NB48. LS mean ± SE; *P<0.001 vs Placebo at all time points. COR‐II: Week 56 data from subjects re‐randomized to NB32 is double‐weighted to account for the pre‐specified exclusion of subjects re‐randomized to NB48. ITT‐LOCF: 38 Subjects with a baseline and ≥1 post‐baseline weight measurement while on study drug. Data on file at Orexigen Therapeutics, Inc. 19 5/15/2015 Improvement in risk factors with use of Naltrexone SR / Bupropion SR Week 28 Measure Placebo N = 456 Week 56 NB32 N = 825 P-value Placebo N = 456 NB32 N = 702 P-value 108.9 ± 11.7 −2.7 ± 0.4 109.3 ± 11.9 −6.2 ± 0.3 <0.001 108.6 ± 11.8 −2.1 ± 0.5 109.0 ± 11.8 −6.7 ± 0.3 <0.001 113.4 ± 1.6 −1.4% (−5.0%, +2.4%) −7.3% (−9.8%, −4.8%) 0.007 112.8 ± 1.6 118.9 ± 1.6 51.4 ± 13.3 +1.2 ± 0.3 <0.001 51.6 ± 12.9 −0.9 ± 0.5 51.8 ± 13.6 +3.6 ± 0.4 <0.001 119.8 ± 30.2 −4.4 ± 0.9 0.004 116.8 ± 32.9 −2.1 ± 1.3 120.5 ± 30.2 −6.2 ± 0.9 0.008 94.8 ± 11.2 −2.1 ± 0.4 0.544 94.2 ± 10.4 −1.3 ± 0.6 95.0 ± 11.3 −2.8 ± 0.5 0.051 10.7 ± 1.9 +3.5% (−3.8%, +11.2%) 11.4 ± 1.9 −11.4% (−15.9%, −6.6%) Waist circumference, cm Baseline Change Triglycerides, mg/dL Baseline Percent change (95% CI) 119.0 ± 1.6 −0.5% (−4.5%, +3.7%) −9.8% (−12.4%, −7.1%) <0.001 HDL-cholesterol, mg/dL Baseline Change 51.4 ± 13.1 −1.4 ± 0.4 LDL-cholesterol, mg/dL Baseline Change 117.1 ± 32.6 0.0 ± 1.3 Fasting blood glucose, mg/dL Baseline Change 94.2 ± 10.4 −1.7 ± 0.5 Fasting insulin, μIU/mL Baseline Percent change (95% CI) 10.7 ± 1.9 −0.5% (−6.5%, +5.9%) 11.4 ± 1.9 −14.1% (−17.9%, −10.2%) <0.001 <0.001 Systolic blood pressure, mm Hg Baseline Change 118.2 ± 10.5 −1.2 ± 0.4 118.1 ± 10.0 −0.9 ± 0.3 0.556 118.2 ± 10.5 −0.5 ± 0.4 117.9 ± 10.0 +0.6 ± 0.3 0.039 76.8 ± 7.0 +0.2 ± 0.2 0.017 76.8 ± 7.0 +0.3 ± 0.3 76.7 ± 7.0 +0.4 ± 0.2 0.847 Diastolic blood pressure, mm Hg Baseline Change 76.8 ± 7.0 −0.7 ± 0.3 Apovian CM, Aronne L, et al. Obesity (Silver Spring). 2013 May;21(5):935-43. 39 Naltrexone/Bupropion: Side Effects Most frequent events: • Nausea • N=171 (29.8%) naltrexone 32 mg plus bupropion • N=155 (27.2%) naltrexone 16 mg plus bupropion • N=30 (5.3%) placebo • Headache, constipation, dizziness, vomiting, and dry mouth were also more frequent in the naltrexone plus bupropion groups vs. placebo • Transient increase of ~1·5 mm Hg in mean systolic and diastolic blood pressure was followed by a reduction of around 1 mm Hg below baseline in the naltrexone plus bupropion groups • Combination treatment was not associated with increased depression or suicides vs. placebo Greenway FL, et al. Lancet. 2010 Aug 21;376(9741):595-605.PMID: 20673995. 40 20 5/15/2015 Liraglutide Approved December 2014 Liraglutide • Glucagon-Like Peptide 1 (GLP-1) receptor agonist approved in 2010 for treatment of type 2 diabetes (1.8 mg/day) • Appetite effect mediated by both the activation of GLP-1 receptors expressed in the hypothalamus • Affects appetite, food preference, and cardiovascular biomarkers in patients with type 2 diabetes • Phase III trials assessing effects of doses as high as 3.0 mg/day submitted to FDA 42 21 5/15/2015 Liraglutide Weight Loss: Two Years Liraglutide 3.0 mg for 1 year (and then maintained on 2.4/3.0 mg for the second year) maintained a mean weight loss of 10.3±7.1 kg from screening over 2 years 3.0 mg 10.3±7.1 kg weight loss Astrup A, et al. Int J Obes (Lond). Jun 2012; 36(6): 843–854. 43 Liraglutide: Adverse Events • Generally well tolerated and improved quality of life • Adverse events mostly mild or moderate • Gastrointestinal events (particularly nausea and vomiting), consistent with the known physiological effects of GLP-1, were more frequent than with placebo • At year 1, nausea and/or vomiting was associated with greater weight loss with liraglutide 3.0 mg, but even those who did not experience these events lost more weight than those on placebo or orlistat • Injection regimen did not impair adherence or cause significant withdrawal during treatment or run-in Astrup A, et al. Int J Obes (Lond). Jun 2012; 36(6): 843–854. 44 22 5/15/2015 Orlistat FDA Approved April 23, 1999 OTC FDA Approved April 23, 1999 Orlistat, Two Year Data 60 or 120 mg TID Selective inhibitor of pancreatic lipase that reduces intestinal digestion of fat; Side effects: initial gastrointestinal symptoms Bray GA, Ryan DH. Ann N Y Acad Sci. 2014 Apr;1311(1):1-13. doi: 10.1111/nyas.12328. 46 23 5/15/2015 Orlistat, 4 Year Data Weight loss (means ± SEM) during 4 years of treatment with orlistat plus lifestyle changes or placebo plus lifestyle changes in obese patients (LOCF data) 3.0 kg 5.8 kg 120 mg Torgerson J S, et al. Dia Care 2004;27:155-161. 47 Medications that Promote Weight Loss/Weight Gain – On/Off Label Type 2 DM and Obesity • Metformin is the first line agent • Exenatide and liraglutide • SGLT-2 inhibitors • Pramlintide Depression and Obesity • Bupropion is the first line agent • Do not use Paxil (choose other SSRIs) Hypertension and Obesity • Do not use B –blockers • Insomnia and Obesity • Do not allow antihistamines 48 24 5/15/2015 Intermittent Versus Continuous Phentermine for Weight Lossa N=64 PBO PHEN (30 mg) PHEN and PBO (alternating every 4 weeks) Mean weight loss, lb 0 -5 -10 -15 -20 -25 -30 -35 0 4 8 12 16 20 Time, weeks 24 28 32 36 Data for completer population (n = 64); patients received only initial instruction regarding diet (simple carbohydrate restriction, ≈ 1000 kcal/d) and reported for follow-up every 4 weeks. a 49 Munro JF, et al. Brit Med J. 1968;1:352-354. Off Label Use : Step Wise Approach Phentermine 15-37.5 mg/d to clinical effect Start at 15 mg or less Topiramate 25-200 mg/d to clinical effect Start at 25 mg afternoon/evening; increase by 25 mg if needed Metformin 500-2000 mg/d to clinical effect Easy to use Can be added to many drugs Bupropion and Naltrexone Bupropion and Zonisamide Liraglutide Exenatide Pramlintide SGLT2 inhibitors ADD: Topiramate ADD: Phentermine ADD: Phentermine (25-200 mg/d to clinical effect) Note: Side effects will be more pronounced than brand name combo phen top Side Effects: Insomnia, anxiety, palpitations (phentermine) and paresthesias and memory impairment (topiramate) Phentermine contraindicated or Side effects from topiramate phentermine and/or metformin 50 25 5/15/2015 On Label Use: Step Wise Approach Phentermine 15-37.5 mg/d to clinical effect Topiramate/phentermine escalate to clinical effect 3.75/23 7/46 11.25/69 15/92 mg Lorcaserin 10 mg BID Bupropion and naltrexone Tablet: 8 mg naltrexone HCl /90 mg bupropion HCl ADD: Phentermine 15 – 37.5 mg Orlistat 120 mg TID Exenatide Injectable for Obese DM Liraglutide Injectable for Obese DM SGLT 2 Inhibitors for Obese DM 51 Summary • New drugs: lorcaserin, phentermine/topiramate, and naltrexone/bupropion • Near approval: liraglutide PDUFA Oct-Dec 2014 • Off label: metformin, bupropion, naltrexone, topiramate, zonisamide, pramlintide, exenatide, liraglutide, SGLT-2 inhibitors • ~5-15% weight loss with newer drugs • Diet and exercise is the cornerstone of weight loss- any diet will achieve weight loss if calorie intake decreased; exercise most important for weight maintenance • Start slow with medications and switch or add if weight loss < 5% in 12 weeks 52 26 5/15/2015 Caroline M. Apovian, MD, FACN, FACP Professor of Medicine and Pediatrics Boston University School of Medicine Director, Center for Nutrition and Weight Management Boston Medical Center 88 East Newton Street Robinson Bldg. Suite 4400 Boston, MA 02118 tel: 617 638-8556 fax:617 638-8599 [email protected] Summary of Randomized Placebo-Controlled Antiobesity Drug Trials Drug 1‐year Δ %WL (mean) % with >5% WL (vs placebo) Frequent Side Effects Uncommon Side Effects 44.1 3.7% 45% vs 20% Dry mouth Fatigue Nausea Urinary tract infection 35.9 43.8 3.0% 47% vs 25% Dizziness Headache Constipation /diarrhea Hypoglycemia (in pts with T2D) 604 (604) 36.0 52.4 3.5% 45% vs 16% 1267 (0) 42.2 42.6 9.4% 67% vs 17% Paresthesia Dry mouth Palpitations Disturbances in attention Constipation Headache Dysgeusia Insomnia Dizziness Alopecia Diarrhea Anxiety and irritability Depression/fatigue Blurred vision Glaucoma BMI Age (mean) (mean) Reference N (T2D) Lorcaserin [Smith et al. 2010] (BLOOM) 3182 (0) 36.2 Lorcaserin [Fidler et al. 2011] (BLOSSOM) 4008 (0) Phentermine/ topiramate [O'Neil et al. 2012] (BLOOM‐DM) [Allison et al. 2012] (EQUIP) Phentermine/ topiramate [Gadde et al. 2011] (CONQUER) 2487 (393) 36.6 51.1 8.6% 70% vs 21% Liraglutide [Astrup et al. 2012] 398 (21) 34.8 45.9 4.9% 73% vs 28% Liraglutide [Wadden et al. 2013] 422 (0) 35.6 46.2 6.1% 51% vs 21% Lorcaserin Nausea Vomiting Constipation Diarrhea Headache Pancreatitis BMI, body mass index; T2D, type 2 diabetes mellitus; WL, weight loss. Manning S, et al. Ther Adv Chronic Dis. 2014;5(3):135-148. 27 5/15/2015 Summary of Naltrexone/Bupropion Drug Trials 56 Weeks of NB32 or Placebo Trial in overweight and obese adults Description Randomized % Subjects in subjects, N completer population(%) Weight loss (%) NB32 COR-I COR-II Subjects with ≥5% weight loss (%) Placebo NB32 8.1 ± 0.5 * 1.8 ± 0.5 * 62% 23% 34% 54% 8.2 ± 0.4 * 1.4 ± 0.5 65% * 22% 39% 793 51% 11.5 ± 0.6 7.3 ± 0.9 80% * 60% 55% 505 54% 5.9 ± 0.5 2.2 ± 0.6 53% * 24% 26% Greenway FL et al Lancet 2010 1742 50% Apovian CM et al Obesity 2013 1496 Wadden T et al Obesity 2011 Wadden T et al Obesity 2011 Placebo Subjects with ≥10% weight loss (%) NB32 Placebo * 11% * 8% * 30% * 8% CORBMOD NB32 plus intensive lifestyle modificatio n * COR-DM overweight and obese adults with type 2 diabetes * Billes SK, et al. Pharmacol Res. 2014 Jun;84:1-11. Anti-obesity Medications in Development Kim GW, et al. Clin Pharmacol Ther. 2014 Jan;95(1):53-66. 28