* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download telomeres and telomerase
History of genetic engineering wikipedia , lookup
Embryonic stem cell wikipedia , lookup
Stem-cell therapy wikipedia , lookup
X-inactivation wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Cellular differentiation wikipedia , lookup
Artificial cell wikipedia , lookup
Induced pluripotent stem cell wikipedia , lookup
Transformation (genetics) wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
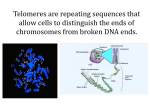
Telomeres and Telomerase: Their Implications for Cancer and Diseases of Aging By: Christina Datuin Elizabeth Blackburn, Ph.D. starts off her presentation by introducing her lab group members by showing pictures of them on her slideshow, then thanking them. She takes this time to acknowledge them for giving her the information she will be discussing in her presentation. Her presentation begins with a statement that says, “Elderly subjects demonstrating exceptional longevity have generally been spared major age-related diseases, such as cardiovascular disease (CD), diabetes mellitus (DM), and cancer, which are diseases that are most responsible for deaths in the elderly.” Her entire presentation is based off of this statement. She introduces telomeres in her discussion. Telomeres are the region of DNA at the ends of eukaryotic chromosomes where a special form of DNA replication occurs. They seal off the ends of chromosomal DNA. Basically, they cap the ends of chromosomes. She goes on to mention the enzyme telomerase. Telomerase is an enzyme that prevents chromosome shortening by attaching many copies of a DNA repeat sequence to the ends of chromosomes. Essentially, telomerase maintains telomeres. She gives an analogy of how telomeres are like the aglets of our genome. Aglets are the little piece of plastic that seal-off the ends of a shoe lace. Telomeres act this way with our genomes in the sense that they cap the ends of them. Telomeres protect the genetic information from leaking out of the genome. She mentioned some research she did on European royal and noble families. She studied them because they are all part of one class and closely related. She found that the longer the parents lived, the longer chance their child had to live. It was discovered that it is a genetic quantitative trait. She started discussing telomeres again. She explained how if you have linear DNA, full replication of the end region cannot occur. The reason for this is because the telomere becomes shorter each time it replicates, so eventually the telomere will be too short to be fully replicated. Each time the cell divides, each chromosome becomes a bit shorter. Basically, telomeres get shorter and shorter until we die of old age. In order to prevent us from dying at a young age, the enzyme telomerase adds a little extra DNA to the end of the chromosome and makes it longer. Before telomerase was discovered, people used to think the shortening of telomeres was the cause for death. Now we know that telomerase prevents that from happening by “turning the hands of the clock” and giving the telomere more time by adding more to it. It does this by copying a little portion within the built-in RNA used for copying nucleotides added to the end of the chromosome, and this elongates the DNA. Basically, telomerase maintains the ends of chromosomes. A full human lifespan requires both telomerase RNA alleles to be functional. Having the correct amount of telomerase is very important and essential. In most cells, things are not in balance. There is a gradual shortening of telomeres. It is assumable that telomerase helps telomeres and cells maintain homeostasis. She mentioned tumors and how they keep replenishing their telomeres. This is a big contribution to how they keep growing. If they keep replenishing their telomeres, they will stay alive and won’t die off, so they will start producing in great amounts. She mentioned how telomerase is on during fetal development and remains active in various proliferative cells. Some of these cells include stem cells, activated lymphocytes, and hair follicles. Telomerase is down-regulated, but still delectable, in many other adult cells. Some of these cells include epithelial, endothelial, and fibroblasts. Telomerase is high in 90% of invasive cancers. It is two-faced in the sense that it is cancerpromoting, while also participating in cell replenishment and telomere protection. These are two extremes, one being very bad and another being very good. If you take telomerase away from cancer cells, there is a small shortening of telomeres that occurs and eventually cells stop multiplying. She compared this to heroin addicts because when addicts quit cold turkey, they experience this sense of shock and their body just comes to a complete halt. The same thing happens to cancer cells when you take their telomerase away. They come to almost a sudden stop in multiplying. Exploiting high telomerase in cancer cells makes toxic telomeric DNA. The high telomerase activity of tumor cells is turned back onto the cells to cause cell death. She then mentioned a rare inherited condition in humans. Premature death from progressive bone marrow failure when one gene copy of telomerase RNA is deficient was shown in multiple pedigrees. Then she mentioned how telomere shortening is implicated in human disease. In arterial endothelial cells, the telomerase shorter by the equivalent of 8 years in heart disease patients versus controls. There were reported people aged 60 years or older in age with shorter blood cell telomeres having higher mortality rates. Shorter telomeres are associated with: 3.2 hold higher mortality rate from heart disease, 3.5 hold higher mortality rate from infectious disease, and pooper survival overall from aggregate of all causes. The mortality rate was worse for people with short telomeres than those with long telomeres. Then she mentioned how she did a study of her own with one of her colleagues. She gave honorable mention to her colleague and praised her. Then she went on to discuss the study design for telomere maintenance. She first showed the psychological stress: 62 healthy premenopausal women (aged 20-50), biological mothers of 1) a healthy child (control mothers) 2) a chronologically ill child (caregiving mothers). They all completed a standardized 10-item questionnaire assessing their level of perveiced stress over the past month. Some questions that were asked had to do with their level of perceived stress and their duration of care-giving. The three markers of cellular aging are telomerase activity, telomere length, and cellular oxidative stress. The results showed that perceived stress all across the whole sample were associated with shorter telmoeres, lower telomerase activity, high oxidative stress, and years of care-giving. The conclusion of her presentation consisted of the final statement that the maintenance of telomeres is something cells take seriously and it is connected to the three main killers in the elderly. Her entire presentation was based off of how telomeres are related to the three main killers in the elderly.