* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Guest Lecture 2: Stem Cell Biology
Survey
Document related concepts
Vectors in gene therapy wikipedia , lookup
Induced stem cells wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Mir-92 microRNA precursor family wikipedia , lookup
Transcript
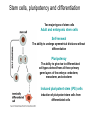
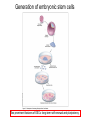
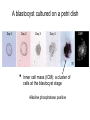
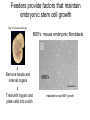
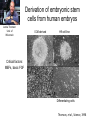
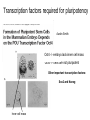
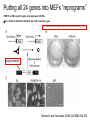
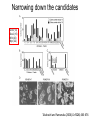
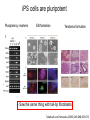
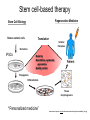
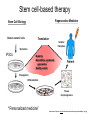
• Overview • Mouse embryonic stem cells • Human embryonic stem cells • Pluripotency genes and network • Long-term self-renewal • Directed differentiation • Induced pluripotent stem cells Stem cells, pluripotency and differentiation Two major types of stem cells Adult and embryonic stem cells Self-renewal The ability to undergo symmetrical divisions without differentiation Pluripotency The ability to give rise to differentiated cell types derived from all three primary germ layers of the embryo: endoderm, mesoderm, and ectoderm Induced pluripotent stem (iPS) cells induction of pluripotent stem cells from differentiated cells Differentiation of human tissues Generation of embryonic stem cells Two prominent features of ESCs: long-term self-renewal and pluripotency A blastocyst cultured on a petri dish Day 1 Day 2 • Day 3 Day 4 Inner cell mass (ICM): a cluster of cells at the blastocyst stage Alkaline phosphatase positive DAPI Isolation of ICM cells Mouse embryos Rabbit Anti-mouse serum Pipetting Outer cells are lysed. Derivation of embryonic stem cells from mouse embryos Martin Evans 2007 Nobel Prize Karyotype is normal Evans, M.J. & Kaufman, M.H. Nature 292, 154-156, 1981 Feeders provide factors that maintain embryonic stem cell growth Day 13 mouse embryos MEFs: mouse embryonic fibroblasts Remove heads and internal organs Treat with trypsin and plate cells into a dish MEFs irradiated to stop MEF growth Embryonic stem cells are pluripotent Embryoid bodies Low attachment ESCs (mixture of differentiated cells) Teratomas Mouse injection Cells of three germ layers Derivation of embryonic stem cells from human embryos Jamie Thomson Univ. of Wisconsin ICM-derived H9 cell line Critical factors: MEFs, basic FGF Differentiating cells Thomson, et al., Science, 1998 What are the promises? • • Understand early human development (infertility, birth defects) and control of cell division (cancer) Cell-based therapy • • Reduce need for organ and tissue donors/transplants Replace mutant or damaged cells for treatment of diseases such as Parkinson’s disease, spinal cord injury, muscular dystrophy, heart disease, liver dysfunction, osteoporosis, vision and hearing loss • A short-cut for drug discovery and testing Transcription factors required for pluripotency Austin Smith Oct4 -/- embryo lack inner cell mass Oct4 -/- cells are not pluripotent Other important transcription factors: Sox2 and Nanog Inner cell mass Core ES cell regulatory circuitry Jaenisch and Young, Cell. 2008 Regulation of long-term self renewal Mouse ESCs LIF (Smith et al., Nature, 1988) BMP (or serum) (Ying et al, Cell, 2003) 3i (Ying et al, Nature, 2008) (Buehr et al, Cell, 2008) LIF and BMP act on downstream differentiation signals of MAPK He S et al. 2009. Annu Rev Cell Dev Biol; Directed ES cell differentiation Transcription factor landscape Graf T and Enver T, 2009, Nature What would be an ideal method for directed differentiation? Rapid Simple Cheap Mimic development Conditions for directed differentiation 1. EBs EB medium hESCs 18 d EB digestion EB formation Hematopoietic stem cells 2. Co-culture OP9 mouse stroma cells – hematopoietic differentiation PA6 or MS5 – neural differentiation 3. Monolayer cultures OP9 coculture Expansion Progenitor Expansion medium 7d Hematopoietic stem cells Neutrophils Terminal differentiation medium 6-7 d Hypothesis Shinya Yamanaka Kyoto University Differentiated somatic cells can be reprogrammed into pluripotent stem (ESClike) cells with gene(s) important for ESC identity (pluripotency and self-renewal) These cells would • Bypass ethical issues • Create patient-specific pluripotent stem cells 24 candidate genes Dppa2 Dppa3/ Stella Dppa4 Dppa5/ Esg1 Ecat1 Ecat3/ Fbx15 Ecat5/ Eras Ecat8 Ecat9/ Gdf3 b-catenin Dnmt3l Fthl17 Grb2 Sox2 Sox15 Tcl1 Oct4 Rex1 Sall4 Utf1 Klf4 Myc Nanog Stat3 Gene delivery: Retrovirus allowing gene integration into the host genome Takahashi and Yamanaka (2006) Cell 126, 663-676 Putting all 24 genes into MEFs “reprograms” FBX15: an ESC-specific gene; only expressed in ESCs bgeo: G418 (an antibiotics that kills the cells) resistance gene So, cells can survive only when they become ESC-like cells Viral promoter Takahashi and Yamanaka (2006) Cell 126, 663-676 Narrowing down the candidates Oct4 (14) Sox2 (15) Klf4 (20) Myc (22) Takahashi and Yamanaka (2006) Cell 126, 663-676 iPS cells are pluripotent Pluripotency markers EB formation Teratoma formation - Saw the same thing with tail-tip fibroblasts Takahashi and Yamanaka (2006) Cell 126, 663-676 How about human cells? • Takahashi K., et al. (2007) “Induction of pluripotent stem cells from adult human fibroblasts by defined factors.” Cell 131, 861-72. • • Yu J., et al. (2007) “Induced pluripotent stem cell lines derived from human somatic cells” Science 318, 1917-1920. • • OCT4, SOX2, KLF4, MYC OCT4, SOX2, NANOG, LIN28 Park I.H., et al. (2007) “Reprogramming of human somatic cells to pluripotency with defined factors” Nature 451, 141-146. • OCT4, SOX2, KLF4, MYC Stem cell-based therapy Regenerative Medicine Stem Cell Biology Human somatic cells Translation Cellular therapies Derivation iPSCs •Scale Up •Quantitative, systematic approaches •Quality control Propagation Differentiation Tissue morphogenesis “Personalized medicine” Adapted from: Gepstein. Circ Res 2002 & http://stemcells.nih.gov/info/media/DSC_1187.jpg Pitfalls with iPSCs • Low efficiency of derivation • Use of C-myc • Transgene integration • Are they really the same as ESCs? <0.1% • Low efficiency of derivation - Are all four genes expressed in the same cells? Approach: Using a single retroviral or lentiviral vector instead of four vectors (2A peptide) Somers A, et al 2010, Stem Cells (STEMCCA Cre-Excisable lentivector) Staerk, J et al, 2010, Cell Stem Cell (T cells and myeloid cells) • Use of C-myc - Chemical complementation (e.g., with small molecules such as VPA) to replace C-Myc Other compounds: Vitamin C, sodium butyrate, ALK5 inhibitor(*, mESC medium), Apigenin and Luteolin (E-cadherin enhancing) Reprogramming with small molecules only? • Transgene integration - integrating-free vectors •Episomal vectors followed by selection of integration free cells •Cre/loxP-recombination system to deliver followed by removal with Cre- recombinase •Single-vector reprogramming system combined with a piggyBac transposon - Protein and mRNA-based •Delivery of OCT-4, SOX2, Myc and Klf4 mRNA or proteins, instead of genes, into somatic cells Protein: polyarginine tag Mouse, 30 days, the need for VPA. Human, 50 days, HEK293 cell extracts Synthetic mRNA: 17 days, 2% efficiency Are iPSCs as good as ESCs? Mouse iPSCs: Can contribute to embryonic development (Takahashi and Yamanaka, Cell, 2006) Produce adult chimera and are germ-line competent (Okita et al, Nature, 2007) Are capable of giving rise to every cell in the new born mice (Zhao et al., Nature, 2009) Journal of Molecular Cell Biology (2010), 2, 171–172 Human iPSCs 1. Global gene expression profiling; 2. Modifications of histone tails; 3. The state of X chromosome inactivation 4. Profiles of DNA methylation At least for some clones, iPSCs are similar if not indistinguishable from ESCs (Mikkelsen et al., Nature, 2008) Stem cell-based therapy Regenerative Medicine Stem Cell Biology Human somatic cells Translation Cellular therapies Derivation iPSCs •Scale Up •Quantitative, systematic approaches •Quality control Propagation Differentiation Tissue morphogenesis “Personalized medicine” Adapted from: Gepstein. Circ Res 2002 & http://stemcells.nih.gov/info/media/DSC_1187.jpg Disease Modeling using iPSCs Disease-specific iPSCs Disease-related differentiated cells Lee, G., Papapetrou, E.P., Kim, H., Chambers, S.M., Tomishima, M.J., Fasano, C.A., Ganat, Y.M., Menon, J., Shimizu, F., Viale, A., Tabar, V., Sadelain, M., and Studer, L. (2009). Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature 461, 402-406. Marchetto, M.C.N., Carromeu, C., Acab, A., Yu, D., Yeo, G. W., Mu, Y., Chen, G., Gage, F.H., and Muotri, A.R. (2010). A Model for Neural Development and Treatment of Rett Syndrome Using Human Induced Pluripotent Stem Cells. Cell, 143, 527-539.