* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The Cosmic Cupboard
Survey
Document related concepts
Definition of planet wikipedia , lookup
IAU definition of planet wikipedia , lookup
Nebular hypothesis wikipedia , lookup
Astrobiology wikipedia , lookup
Stellar evolution wikipedia , lookup
History of Solar System formation and evolution hypotheses wikipedia , lookup
Extraterrestrial atmosphere wikipedia , lookup
Observational astronomy wikipedia , lookup
Formation and evolution of the Solar System wikipedia , lookup
Planetary habitability wikipedia , lookup
Astronomical spectroscopy wikipedia , lookup
Extraterrestrial life wikipedia , lookup
Hypothetical types of biochemistry wikipedia , lookup
Transcript
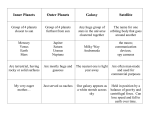
The Cosmic Cupboard •How do astronomers know what elements are in the universe to make planets from? •What is the cosmic abundance of elements? •What molecules will result from this cosmic abundance? •How will these materials sort themselves around a young star? Radio Telescopes can detect the spectral signature of elements across the universe. • Natural radio emission from elements can travel vast distances. • Terrestrial radio telescopes are very sensitive. • Searches for elements in the interstellar medium and in external galaxies have been made. Receiver This is a typical stand alone radio telescope •Natural radio emission is collected by the dish •The disk reflects the radio ways and concentrates them at the receiver. Control room in Trailer •The receiver further amplifies the signal and passes it to the control room where astronomers are looking at the data. This array of Radio Telescopes in New Mexico has 21 separate radio telescopes that can be operated independently or electronically arrayed together to act as one giant radio telescope of unsurpassed resolution This is an optical image of Jupiter This is a radio image of Jupiter. The radio images shows a band of emission around the equatorial region similar to the Van Allen Belts around the Earth. This is an optical image of an elliptical galaxy called NGC 6251 Radio Lobes Radio Jets This is a radio image of the same elliptical galaxy NGC 625. The visual galaxy does not appear. Instead, two large lobes of radio emission appear from jets that are This is a combined optical and Radio image. The point is to show you that the radio telescopes can detect structures that are not visible in ordinary optical telescopes. This 13 mm spectrum of the molecular cloud SgrB2(N) near the Galactic center is completely dominated by molecular lines from known and unknown (U) species (Ziurys et al. 2006, NRAO Newsletter, 109, 11). More than 140 different molecules containing up to 13 atoms (HC11N) have been identified in space. Spectrum of NGC 3783 (black). The most important spectral features in the data and model are labelled. The Cosmic Abundance of Elements • Hydrogen is the overwhelmingly most abundant element in the universe – 87.6% • Helium is next in abundance – 12.3% • These two elements comprise 99.9% of the atoms in the universe. • All other elements are in very low abundance. Most abundant elements, H2 and He 100 times less abundant elements, C, N, O & Ne 100 times less abundant again Trace abundances Cosmic Abundance of the Elements hydrogen helium oxygen carbon neon nitrogen 10,000,000 1,400,000 6,800 3,000 2,800 910 magnesium 290 silicon 250 95 sulfur 80 iron 42 argon aluminum 19 17 sodium 17 calcium all other 50 elements . . Abundance of Molecules in the Universe • In space, molecules are formed by collisions between atoms. • The most common molecules will be formed from atoms that are most likely to collide with each other. • The Nobel gases Helium and Neon will not form bonds with other elements. Examine this list of cosmic abundances. What molecules Cosmic Abundance of (combinations of elements) are likely to form from the Elements random collisions in a mixture of these gases? hydrogen helium oxygen carbon neon nitrogen 10,000,000 1,400,000 6,800 3,000 2,800 910 magnesium 290 silicon 250 sulfur iron argon aluminum sodium calcium all other elements . 95 80 42 19 17 17 50 . Ignore Helium an Neon because these are Inert gases that Cosmic Abundance of will not form any molecules (except under some very the Elements artificial circumstances in the laboratory. hydrogen helium oxygen carbon neon nitrogen 10,000,000 1,400,000 6,800 3,000 2,800 910 magnesium 290 silicon 250 sulfur iron argon aluminum sodium calcium all other elements . 95 80 42 19 17 17 50 . IfCosmic two atoms were to “bump” into each other in this mixture, Abundance of what two atoms would they be? Since Hydrogen represents the overwhelmingthe majority Elements of atoms, the two would be H and they would form a molecule H2, molecular hydrogen. hydrogen helium oxygen carbon neon nitrogen 10,000,000 1,400,000 6,800 3,000 2,800 910 magnesium 290 silicon 250 sulfur iron argon aluminum sodium calcium all other elements . 95 80 42 19 17 17 50 . What molecule would form next? That is, after H-H collisions, what would be the next most common collision? itof would be What molecule would form next? That is, afterClearly H-H collisions, Cosmic Abundance between hydrogen H2O. Water is the Clearly second it most what wouold be the and nextoxygen, most common collision? the Elements abundant in the Universe. Water H is2O. everywhere would bemolecule between hydrogen and oxygen, Water is (in the some form). in the Universe. second most abundant molecule hydrogen helium oxygen carbon neon nitrogen 10,000,000 1,400,000 6,800 3,000 2,800 910 magnesium 290 silicon 250 sulfur iron argon aluminum sodium calcium all other elements . 95 80 42 19 17 17 50 . We could continue this “collisional” analysis, looking at Cosmic Abundance what molecules would be the next most common, butof I’d rather just present results and let you see that nature has thethe Elements made or job of understanding what goes into making a planet a bit simpler that we may have though. hydrogen helium oxygen carbon neon nitrogen 10,000,000 1,400,000 6,800 3,000 2,800 910 magnesium 290 silicon 250 sulfur iron argon aluminum sodium calcium all other elements . 95 80 42 19 17 17 50 . The most common molecules in space that planets are constructed from begins with … • Molecular Hydrogen and Helium – Helium is not really a molecule but we will count it now because of its high abundance. – These two GASES represent the overwhelming amount of material that stars and planets form from. Next, we find a class of molecules we will call ICES • • • • • Water, H2O Methane, CH4 Ammonia, NH3 Carbon Dioxide, CO2 These molecules are solid when cold, but will vaporize when warmed. Thus the moniker “Ices” Finally, we come to the last class of molecules that we will collectively call “Rock” • Quartz, SiO4 • Silicate Minerals (SiO3+ (Fe, Mg, AL, etc..) – are the common minerals that make up the igneous rocks of the Earth. • Metallic Iron, Fe • Metallic Nickle, Ni • These molecules are solid when cold, and remain solid unless heated to exceptionally high temperatures. Thus, we will consider them to be always solid. The Cosmic Cupboard • We have clearly oversimplified the chemistry occurring in the cosmos. However, we have not deviated from its true outcome. • There are three basic ingredients available to built planets – Gas (H2, He) – Ices (H2O, CH4, NH3, CO2) – And Rock (Silicate Minerals, Iron and Nickle) The Cosmic Cupboard • Gas (H2, He) is the overwhelmingly abundant material. • Ices (H2O, CH4, NH3, CO2) are perhaps 100 times less abundant than gases, and • Rock (Silicate Minerals, Iron and Nickel) is 100 times less abundant than Ices. Imagine the following cupboard of ingredients from which you can make a planet… 10,000 Parts GAS 100 Parts ICE 1 Part Rock GAS Let’s make a simple deduction. Why are there no giant planets in our Solar System made entirely of Rock. In other words why do we not see any Jupiter sized Terrestrial Planets? Obviously, there is not enough rock available. You cannot make a giant planet out of a tiny container of rock. Thus we can understand why the Terrestrial planets are so small. They are made of the least abundant material! ICE Rock How will this material sort out around a young star? GAS ICE Rock The Distribution of Materials in the Solar Nebula 10,000 Amount of Material Gas is everywhere and most abundant Ice is next in abundance 100 Rock is least in abundance 1 Distance from the Proto-Sun The Distribution of Materials in the Solar Nebula Amount of Material 10,000 Ices too close to the Proto-Sun evaporate and become gases. Thus, solid ices begin only beyond a distance from the Proto-Sun we will call the Ice Line. 100 1 Distance from the Proto-Sun Underlying Planet Formation Facts • All planets begin forming by an accumulation of solid material. • Close to the Sun only rock is available as a solid to form planetesimals. • Far from the Sun ices constitute the vast bulk of solid material and icy planetesimals are common. • There is hundreds of times more solid ice than rock. The reservoir of solid material to initiate planet formation is much larger when ices are solid. The Distribution of Materials in the Solar Nebula Amount of Material 10,000 Planets inside the Ice Line can only be small and rocky. There is not enough rock and the gas is too hot for them to accrete the Hydrogen and Helium around them. Thus the planets in close are small and rocky. 100 1 Ice Line Distance from the Proto-Sun Planets beyond the Ice Line have a much larger reservoir of solid material to use. They have Ice as well as Rock. There is a 100 times the amount of Ice compared to Rock. So the planets that form beyond the Ice Line start with much larger planetary cores of Ice and Rock. These large cores have enough gravity to accrete to cold hydrogen and helium around them. Thus they grow to be gas giant planets, even though they started as mostly Ice and some rock cores. The Distribution of Materials in the Solar Nebula Amount of Material 10,000 100 1 IceLine Distance from the Proto-Sun The Distribution of Materials in the Solar Nebula Amount of Material 10,000 Further, we can now see why Jupiter is the largest Jovian planet because it had the largest reservoir of solid material to form from and was able to gather the most gas. The succeeding Jovian planets all get smaller as the reservoir of material diminishes. 100 1 Ice Line Distance from the Proto-Sun