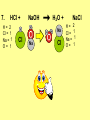
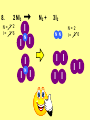
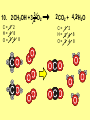
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Physical organic chemistry wikipedia , lookup
Marcus theory wikipedia , lookup
Electrochemistry wikipedia , lookup
Electrolysis of water wikipedia , lookup
Biochemistry wikipedia , lookup
Chemical reaction wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Atomic theory wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
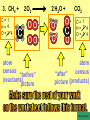
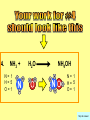
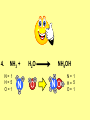
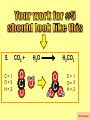
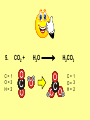
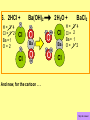
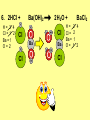
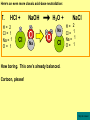
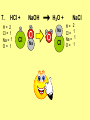
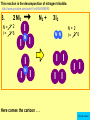
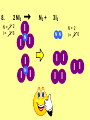
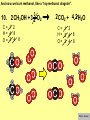
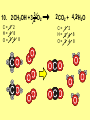
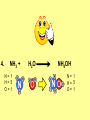
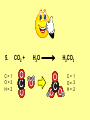
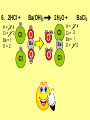
Visualizing the balancing of chemical equations By Daniel R. Barnes Init: 1/9/2007 from previous work You may hit the “End” key at any time to jump to the hyperTable of Contents. NOTE: This presentation is meant to go along with Mr. Barnes’ cardboard disc reactions worksheet, which is meant to be used with a set of specially-colored cardboard discs, each disc representing an atom of an element indicated by the color of the disc. NOTE: As always, some of the images in this presentation have been taken from the world wide web without permission of their owners. Copying and distribution of this presentation may be, therefore, illegal. In fact, its mere existence may be illegal. SWBAT . . . . . . balance chemical equations. . . . explain why some reactions are endothermic and other reactions are exothermic. . . . define “activation energy” and give real life examples. reactants 1. C+ turn into O2 products CO2 Skip to answer 1. C+ O2 CO2 Skip to answer 1. C+ O2 CO2 Skip to answer 1. C+ O2 CO2 Please note: charcoal briquette ashes are not made of carbon dioxide. CO2 is a colorless, odorless gas. Charcoal ashes are made of alkali metal compounds and other stuff. Charcoal is not pure carbon. If it were, there would be no ashes left after charcoal burned. Skip to answer 1. C= 1 O= 2 C+ O2 CO2 C= 1 O= 2 Before you go to your lab tables and start working with the discs, we’d better make sure we know the definitions of . . . Chemicals absorb energy Surroundings get colder. Chemicals release energy Surroundings get hotter. All chemical reactions, whether they are endothermic or exothermic, typically involve endothermic bond breakage AND exothermic bond formation. If forming new bonds releases more energy than the activation energy required to break the old bonds . . . . . . The reaction will be EXOTHERMIC If forming new bonds releases less energy than the activation energy required to break the old bonds . . . . . . The reaction will be ENDOTHERMIC Sell $100 worth of hot dogs Sell $400 worth of hot dogs Spend $200 for materials Spend $200 for materials $100 LOSS $200 PROFIT Yields money Yields energy Sell $100 worth of hot dogs Making new bonds Sell $400 worth of hot dogs Spend $200 for materials Breaking old bonds Spend $200 for materials $100 LOSS Costs money $200 PROFIT Costs energy NOTE: The circled numbers are made up. They’re not real. Relase 100 kJ of energy forming new bonds Absorb 200 kJ of energy breaking old bonds ENDOthermic Making new bonds Breaking old bonds Release 400 kJ of energy forming new bonds Absorb 200 kJ of energy breaking old bonds EXOthermic Q: What is the difference between an endothermic process and an exothermic process? A: Endothermic processes absorb heat, making their surroundings colder, whereas exothermic processes give off heat, making their surroundings get hotter. Q: What is activation energy? A: The energy input required to make a reaction happen, even if it’s an exothermic reaction. It’s the energy required to break old bonds. Mr. Barnes, please (1) Open the windows. (2) Put goggles on the kids in the front two rows. (3) Put a few drops of glycerine on a dimpled pile of potassium permanganate. This reaction is definitely exothermic, as shown by the fire coming out of it. The reaction was spontaneous at room temperature. No matches, sparks, UV light, or other input of activation energy was needed to get it going. The apparently low activation energy may indicate weak bonds somewhere in the reactants. One of the main reactions that happens when these two chemicals go crazy on each other is the following: 14KMnO4(s) + 4C3H5(OH)3(l) → 7K2CO3(s) + 7Mn2O3(s) + 5CO2(g) + 16H2O(g) I’ve left the coefficients covered so that you can try to balance this monster for extra credit when you’re all done with the worksheet. A slide near the end of this presentation has the coefficients on it if you want to find out what they are. 1. C+ O2 C= 1 O= 2 CO2 C= 1 O= 2 Your worksheet should already look like this for #1. Mr. Barnes will now have you animate the reaction with cardboard discs. At this point, Mr. Barnes may wish to turn off the projector. If he does, the rest of this presentation can be viewed at hhscougars.org, on Mr. Barnes’ Chemistry Power Points page. 2. H2 + O2 H2O formulas subscripts Skip to answer 2. H2 + O2 H2O Explosive chemical reactions can blow up a vehicle and destroy it, but they can also make vehicles move in the first place. The big, orange external fuel tank of the space shuttle contains hydrogen and oxygen. The two gasses are mixed together and ignited in the three engines under the tail fin of the orbiter. The flames that come Skip to answer out of those engines are made The white rockets on the side of of water vapor – water vapor the fuel tank use a solid fuel. I that’s so hot that it glows. don’t know what that fuel is. 2 H2 + 2. H=2 4 O=2 O2 2 H2O H =2 4 O =1 2 coefficient InIf ayou chemical reaction, start out with two atoms are neither oxygen atoms, you created nor destroyed. should end up with two They justatoms change oxygen who they’re stuck to. Okay. Let’s re-run the reaction from the beginning, but this time we’re going to get it right the first time. Skip to answer 2. H=2 4 O=2 2 H2 + O2 2 H2O H =2 4 O =1 2 Skip to answer 2. H=2 4 O=2 2 H2 + O2 2 H2O H =2 4 O =1 2 Skip to answer 2. H=2 4 O=2 2 H2 + O2 2 H2O H =2 4 O =1 2 3. CH4 + C= 1 H= 4 O=2 O2 H 2O + CO2 C= 1 H= 2 O= 3 First, we need to see if the equation is balanced or not. We need to do an . . . atom count. Skip to answer 3. CH4 + C= 1 H= 4 O=2 4 2 O2 2 H 2O + CO2 C= 1 H= 2 4 O= 3 4 Something is obviously wrong. Let’s animate the equation as it is and see if we notice anything weird. Skip to answer 3. CH4 + 2 O2 2 H 2O + CO2 Skip to answer 3. CH4 + C= 1 H= 4 O=2 4 2 O2 2 H 2O + CO2 C= 1 H= 2 4 O= 3 4 Skip to answer 3. CH4 + 2 O2 C= 1 H= 4 O=2 4 atom census “before” (reactants) picture 2 H 2O + CO2 C= 1 H= 2 4 O= 3 4 atom census “after” picture (products) Skip to answer 3. CH4 + C= 1 H= 4 O=2 4 2 O2 2 H 2O + CO2 C= 1 H= 2 4 O= 3 4 The famous “ammonia fountain” demonstration is something we won’t explain until chapter 14 (gases). However, if you want to see it happen, click the link below. If that doesn’t work, just go to YouTube and type in “ammonia fountain.” http://www.youtube.com/watch?v=4U-DgdWPKyo 4. NH3 + N= 1 H= 5 O=1 H2O NH4OH N= 1 H= 5 O= 1 Skip to answer The famous “ammonia fountain” demonstration is something we won’t explain until chapter 14 (gases). However, if you want to see it happen, click the link below. If that doesn’t work, just go to YouTube and type in “ammonia fountain.” http://www.youtube.com/watch?v=4U-DgdWPKyo 4. NH3 + N= 1 H= 5 O=1 N H2O NH4OH N N= 1 H= 5 O= 1 Skip to answer 4. NH3 + N= 1 H= 5 O=1 N H2O NH4OH N N= 1 H= 5 O= 1 Skip to answer 4. NH3 + N= 1 H= 5 O=1 N H2O NH4OH N N= 1 H= 5 O= 1 When water and carbon dioxide mix, they produce carbonic acid. Carbonic acid is a “weak” acid found in soda and any other carbonated beverage. It may be a “weak” acid, but it is acidic enough to partially dissolve certain rocks over time. Carbonic acid, therefore, is an agent of “chemical weathering”. 5. C= 1 O=3 H= 2 CO2 + H2O H2CO3 C= 1 O= 3 H= 2 Skip to answer When water and carbon dioxide mix, they produce carbonic acid. Carbonic acid is a “weak” acid found in soda and any other carbonated beverage. It may be a “weak” acid, but it is acidic enough to partially dissolve certain rocks over time. Carbonic acid, therefore, is an agent of “chemical weathering”. 5. C= 1 O=3 H= 2 CO2 + H2O H2CO3 C= 1 O= 3 H= 2 Skip to answer 5. C= 1 O=3 H= 2 CO2 + H2O H2CO3 C= 1 O= 3 H= 2 Skip to answer 5. C= 1 O=3 H= 2 CO2 + H2O H2CO3 C= 1 O= 3 H= 2 The reaction below is an example of an acid-base “neutralization”. 6. HCl + Ba(OH)2 H= 3 Cl = 1 Ba = 1 O= 2 H 2O + H= Cl = Ba = O= BaCl2 2 2 1 1 You might be tempted to try to balance hydrogen first, because it’s the first element in the equation, but I wouldn’t if I were you. Notice that hydrogen appears in three different formulas in the equation. Trust me when I say that you should balance first those elements that appear in the least number of formulas. Let’s save hydrogen for later . . . Skip to answer 6. HCl + Ba(OH)2 H= 3 Cl = 1 Ba = 1 O= 2 H 2O + H= Cl = Ba = O= BaCl2 2 2 1 1 The second element in the equation, chlorine, appears in only two formulas (the smallest possible # of places). Let’s balance chlorine first. We balance an element by increasing whatever coefficient will increase the amount of that element. Remember: when you’re balancing an equation you can only change the coefficients. DON’T TOUCH THE SUBSCRIPTS! Skip to answer 6. HCl 2+ Ba(OH)2 H 2O + BaCl2 H= 2 H= 3 Cl = 2 Cl = 1 Ba = 1 Ba = 1 O= 1 O= 2 EXAMPLE: Someone who didn’t know what they’re doing might foolishly put a “2” as a subscript after the “Cl” in “HCl”. DON’T DO THAT! You’re not supposed to change subscripts when you balance a chemical equation! There’s no such chemical as HCl2, and even if there were, it wouldn’t be hydrochloric acid anymore. When you balance a chemical equation, you’re not changing the identity of the chemicals in the reaction, just how much of Skip to answer each chemical there is. 6. 2HCl + H= 3 4 Cl = 1 2 Ba = 1 O= 2 Ba(OH)2 H 2O + H= Cl = Ba = O= BaCl2 2 2 1 1 The proper thing to do is to put a coefficient of “2” in front of HCl on the left. As soon as you change a coefficient, you need to adjust the atom count for any element in that formula. Doubling the amount of HCl increases the amount of H . . . . . . and also the amount of Cl. We’ve now balanced the # of Cl’s on the left and the right. Skip to answer 6. 2HCl + Ba(OH)2 H= 3 4 Cl = 1 2 Ba = 1 O= 2 2 H 2O + H= Cl = Ba = O= BaCl2 2 4 2 1 1 2 Now chlorine is fixed, but there still isn’t enough oxygen on the right. Putting a coefficient of “2” in front of H2O on the right will double the amount of oxygen, balancing it as well. Of course, the “2” doubles not only the O’s on the right, but , but also the H’s. Would you look at that? The H’s got balanced by accident! Skip to answer 6. 2HCl + H= 3 4 Cl = 1 2 Ba = 1 O= 2 Ba(OH)2 2 H 2O + Cl Cl Ba Cl Ba H= Cl = Ba = O= BaCl2 2 4 2 1 1 2 Cl And now, for the cartoon . . . Skip to answer 6. 2HCl + H= 3 4 Cl = 1 2 Ba = 1 O= 2 Ba(OH)2 Cl Cl Ba Cl 2 H 2O + Ba Cl H= Cl = Ba = O= BaCl2 2 4 2 1 1 2 Here’s an even more classic acid-base neutraliztion: 7. HCl + H= 2 Cl = 1 Na = 1 O= 1 NaOH H 2O + Na Cl Na Cl NaCl H= 2 Cl = 1 Na = 1 O= 1 How boring. This one’s already balanced. Cartoon, please! Skip to answer 7. HCl + H= 2 Cl = 1 Na = 1 O= 1 NaOH H 2O + Na Cl Na Cl NaCl H= 2 Cl = 1 Na = 1 O= 1 This reaction is the decomposition of nitrogen triiodide. http://www.youtube.com/watch?v=2KlAf936E90 8. 2 NI3 N2 + 3 I2 N= 1 2 I= 3 6 N= 2 I= 2 6 There’s not enough nitrogen on the left, so we need to get more by . . . . . . putting a coefficient of “2” in front of NI3. As always, any time you change a coefficient, you must adjust the atom counts for every element in the formula you put the coefficient in front of. Now we need more iodine on the right, so . . . Skip to answer This reaction is the decomposition of nitrogen triiodide. http://www.youtube.com/watch?v=2KlAf936E90 8. 2 NI3 N2 + I N= 1 2 I= 3 6 I N I I N N I I I N 3 I2 I N= 2 I= 2 6 I I I I Here comes the cartoon . . . Skip to answer 8. 2 NI3 N2 + I N= 1 2 I= 3 6 I N I I N N I I I N 3 I2 I I I N= 2 I= 2 6 I I The following reaction is steel wool burning. http://www.youtube.com/watch?v=39diUfWnbPY 9. 2 Fe + 3 2 O2 Fe = 1 2 O= 2 3 Fe2O3 Fe = 2 O= 3 There’s not enough iron on the left, so . . . Now, we’ve got a problem. We want to end up with three oxygen atoms on the left, but if we put a “2” in front of the “O2” on the left, we’ll end up with four “O’s” on the left instead of three. 1O2 is not enough, and 2O2 is too much. The solution is a little crazy. We can put a 3/2 as a coefficent. 3/2 x 2 = 6/2 = 3. Now we’re balanced. It’s actually standard procedure for chemists to use fractional coefficients, but only if it’s an odd number over two, and only Skip to answer if it’s in front of a diatomic element. The following reaction is steel wool burning. http://www.youtube.com/watch?v=39diUfWnbPY 9. 4 2 Fe + 3 Fe = 1 2 4 O= 2 3 6 3 2 O2 2 Fe2O3 Fe = 2 4 O= 3 6 We’ve got a problem when it comes time to draw the cartoon for the reaction, though. Drawing 3/2 of an oxygen molecule is kind of like drawing 3/2 of a person or 3/2 of a tv. The solution is to double all the coefficients in the equation. Doubling all the coefficients doubles all the atom counts . . . . . . and the equation is still just as balanced as before the doubling. Skip to answer The following reaction is steel wool burning. http://www.youtube.com/watch?v=39diUfWnbPY 9. 4 2 Fe + 3 Fe = 1 2 4 O= 2 3 6 Fe 3 2 O2 2 Fe2O3 Fe Fe Fe Fe Fe = 2 4 O= 3 6 Fe Fe Fe Now that all the coefficients are whole numbers, drawing the cartoon will be much easier. We won’t have to split any circles in half or anything dumb like that. Skip to answer 9. 4 2 Fe + 3 Fe = 1 2 4 O= 2 3 6 Fe 3 2 O2 2 Fe2O3 Fe Fe Fe Fe Fe = 2 4 O= 3 6 Fe Fe Fe And now, we burn methanol, like a “top methanol dragster”. 10. CH3OH + O2 C= 1 H= 4 O= 3 CO2 + H 2O C= 1 H= 2 O= 3 Notice that oxygen occurs in all four formulas in this equation. We should save oxygen for last and balance something else first. Carbon is already balanced, and hydrogen appears in only two places, so hydrogen should be our first element to balance. Skip to answer And now, we burn methanol, like a “top methanol dragster”. 10. CH3OH + O2 C= 1 H= 4 O= 3 CO2 + 2 H 2O C= 1 H= 2 4 O= 3 4 We’re almost balanced now, but we need one more oxygen atom on the left. Unfortunately, if we add an O2, we get two more oxygen atoms, not one more. Don’t you worry! Oxygen is a diatomic element, so we can use fractional coefficients with O2, as long as the fraction is an odd number divided by two. Skip to answer And now, we burn methanol, like a “top methanol dragster”. 10. 2 CH3OH + 3 23 O2 2 CO2 + 4 2 H2O C= 1 2 C= 1 2 H= 4 8 H= 2 4 8 O= 3 4 8 O= 3 4 8 Now we’re balanced, and most chemists would say we’re done. However, we want to draw a cartoon, and half-circles look stupid, so let’s get rid of that fraction by . . . . . . multiplying all coefficients by two. This means multiplying all atom counts by two. Now we’re ready to draw the cartoon. Skip to answer And now, we burn methanol, like a “top methanol dragster”. 10. 2 CH3OH + 3 23 O2 C= 1 2 H= 4 8 O= 3 4 8 2 CO2 + 4 2 H2O C= 1 2 H= 2 4 8 O= 3 4 8 Skip to answer 10. 2 CH3OH + 3 23 O2 C= 1 2 H= 4 8 O= 3 4 8 2 CO2 + 4 2 H2O C= 1 2 H= 2 4 8 O= 3 4 8 Dang. You made it this far? E-mail Mr. Barnes and tell him that you saw the sasquatch if you want some XCR. I bet you need more in-class practice. You need to balance some more equations. Your group leader needs to get everyone in your group an “SP Book”. YOUR ASSIGNMENT: Section 9:2 pg 64: Section 9:3 pg 65: pg 66: pg 67: PROBLEMS 1-5 PROBLEMS 11 – 14 PROBLEMS 21 – 24 PROBLEMS 31 – 34 The answers are in the back of the book, on pages 273-274 (You can do the other problems in 9:2 and 9:3 for extra credit if you’re really brave, but they might be hard.) What do the following things mean? (cr) = “crystal” (s) = “solid” (l) = “liquid” (g) = “gas” (aq) = “aqueous” = dissolved in water They all have something to do with . . . states of matter. Please ignore them in this exercise. They don’t affect equation-balancing at all. EQUATION-COPYING TIPS: (i) Don’t copy state of matter info ((cr), (s), (l), (g), or (aq)). (ii) Leave plenty of room in front of each formula so that you can put coefficients there. Consider that you might have to change a coefficient more than once, so leave plenty of room. For example, if the SP book says “1. Mg(cr) + O2(g) MgO(cr)” You should write, on your paper, “1. LOTS OF Mg SPACE LOTS + OF O2 SPACE LOTS OF MgO” SPACE One last thing . . . Please remember the following saying: Give a man a fish, and you feed him for a day. Teach a man to fish, and you feed him for a lifetime. There’s only one teacher in the room, and I might be busy when you need help. If you run into trouble, ask the people around you for help. If you do, don’t just ask for the answer. Ask HOW you get the answer. now “SP Book Problems, 9:2 & 9:3” YOUR ASSIGNMENT: Section 9:2 pg 64: Section 9:3 pg 65: pg 66: pg 67: PROBLEMS 1-5 PROBLEMS 11 – 14 PROBLEMS 21 – 24 PROBLEMS 31 – 34 The answers are in the back of the book, on pages 273-274. If you get one right, put a happy face. If you get it wrong but understand it after you see the answer, put a check. If you still don’t get it even after you see the answer, put a question mark and get help from a neighbor or your teacher. Have you tried balancing the equation below yet? 14KMnO4(s) + 4C3H5(OH)3(l) → 7K2CO3(s) + 7Mn2O3(s) + 5CO2(g) + 16H2O(g) Don’t press any buttons until you’ve tried, or you’ll spoil the surprise. Did you get it right? If you couldn’t, you might ask Mr. Barnes to show you his algebraic method for balancing difficult equations. 1. C= 1 O= 2 C+ O2 CO2 C= 1 O= 2 2. H=2 4 O=2 2 H2 + O2 2 H2O H =2 4 O =1 2 3. CH4 + C= 1 H= 4 O=2 4 2 O2 2 H 2O + CO2 C= 1 H= 2 4 O= 3 4 4. NH3 + N= 1 H= 5 O=1 N H2O NH4OH N N= 1 H= 5 O= 1 5. C= 1 O=3 H= 2 CO2 + H2O H2CO3 C= 1 O= 3 H= 2 6. 2HCl + H= 3 4 Cl = 1 2 Ba = 1 O= 2 Ba(OH)2 Cl Cl Ba Cl 2 H 2O + Ba Cl H= Cl = Ba = O= BaCl2 2 4 2 1 1 2 7. HCl + H= 2 Cl = 1 Na = 1 O= 1 NaOH H 2O + Na Cl Na Cl NaCl H= 2 Cl = 1 Na = 1 O= 1 8. 2 NI3 N2 + I N= 1 2 I= 3 6 I N I I N N I I I N 3 I2 I I I N= 2 I= 2 6 I I 9. 4 2 Fe + 3 Fe = 1 2 4 O= 2 3 6 Fe 3 2 O2 2 Fe2O3 Fe Fe Fe Fe Fe = 2 4 O= 3 6 Fe Fe Fe 10. 2 CH3OH + 3 23 O2 C= 1 2 H= 4 8 O= 3 4 8 2 CO2 + 4 2 H2O C= 1 2 H= 2 4 8 O= 3 4 8 Title Page 5. SP Book SWBATS 6. Demo EQ answer 1. 7. 2. 8. Just the answers 3. 9. 4. 10.