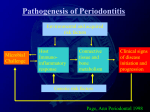
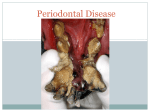
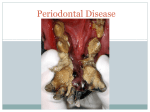
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Systematic Review and Meta-Analysis on the Nonsurgical Treatment
Gene therapy wikipedia , lookup
Focal infection theory wikipedia , lookup
Transtheoretical model wikipedia , lookup
Dental avulsion wikipedia , lookup
Clinical trial wikipedia , lookup
Epidemiology wikipedia , lookup
Forensic epidemiology wikipedia , lookup
Dental emergency wikipedia , lookup
Management of multiple sclerosis wikipedia , lookup
Systematic Review and Meta-Analysis on the Nonsurgical Treatment of Chronic Periodontitis by Scaling and Root Planing with or without Adjuncts ABSTRACT Background A panel of experts convened by the American Dental Association (ADA) Council on Scientific Affairs (CSA) presents a systematic review and meta-analysis on nonsurgical treatment of patients with chronic periodontitis by scaling and root planing (SRP) with or without adjuncts. Types of studies reviewed The authors conducted a search of PubMed/MEDLINE and Embase for randomized controlled trials of SRP with or without the use of adjunctive treatments with clinical attachment level (CAL) outcomes of trials at least six months in duration and published in English through July 2014. Results The panel included 72 articles in its review and assessed the level of certainty in as well as efficacy of SRP with or without systemic antimicrobials, a systemic host modulator (sub-antimicrobial dose doxycycline), locally-delivered antimicrobials (chlorhexidine chips, doxycycline hyclate gel, and minocycline microspheres), and a variety of lasers used nonsurgically (photodynamic therapy [PDT] with a diode laser, a diode laser [non-PDT], Nd: YAG lasers, and erbium lasers). The panel also presents evidence on the potential adverse events associated with each treatment.The panel makes recommendations for further research. Conclusions The literature on randomized controlled trials of SRP versus no treatment or debridement is scant, but confirmed the commonly reported result of approximately 0.5 mm improvement in CAL. The literature on adjunctive therapies was varied providing only a moderate level of certainty on the benefits of the four adjunctive therapies: systemic sub-antimicrobial dose doxycycline, systemic antimicrobials, chlorhexidine chips, and photodynamic therapy with a diode laser. There was a low level of certainty on the benefits of all other adjunctive therapies. The panel also assessed the balance between the benefits and potential for adverse events and harms from each treatment. The panel makes specific recommendations for additional research on the topic of nonsurgical treatment for chronic periodontal disease to fill the gaps in knowledge and improve the evidence base. Clinical implications The associated Clinical Practice Guideline summarizes the clinical implications of this systematic review. July 2015 Page 1 AUTHORS Christopher J. Smiley, DDS; Sharon L. Tracy, PhD; Elliot Abt, DDS, MSc, MS; Bryan Michalowicz, DDS; Mike T. John, Dr. med. dent., PhD, MPH; John (Jack) Gunsolley, DDS, MS; Charles M. Cobb, DDS, PhD; Jeffrey Rossmann, DDS, MS; Stephen K. Harrel, DDS; Jane L. Forrest, EdD; Philippe P. Hujoel, DDS, MSD, MS, PhD; Kirk W. Noraian, DDS, MS, MBA; Henry Greenwell, DMD, MSD; Julie Frantsve-Hawley, PhD; Cameron Estrich, MPH; Nicholas (Buck) Hanson, MPH AFFILIATIONS Christopher J. Smiley is a dentist in private practice in Grand Rapids, MI. He was the chair of the panel. Sharon L. Tracy is assistant director, Center for Evidence-Based Dentistry, Division of Science, American Dental Association, Chicago, IL. Elliot Abt is Attending Staff, Department of Dentistry, Advocate Illinois Masonic Medical Center, Chicago, IL. Bryan Michalowicz is Professor and Erwin Schaffer Chair in Periodontal Research, Division of Periodontology, Department of Developmental and Surgical Sciences at the University of Minnesota. Mike T. John is Associate Professor in the Division of TMD and Orofacial Pain, Department of Diagnostic and Biological Sciences at the University of Minnesota. John (Jack) Gunsolley is Professor of Periodontics at Virginia Commonwealth University. Charles M. Cobb is Interim Director, Advanced Program, and Professor Emeritus, Department of Periodontics at the University of Missouri-Kansas City in Kansas City, MO. He represented the American Academy of Periodontology on the panel. Jeffrey Rossmann is Professor and Chair of the Department of Periodontics at Texas A&M University Baylor College of Dentistry, Dallas, TX. Stephen K. Harrel is Adjunct Professor of Periodontology at Texas A&M University Baylor College of Dentistry, Dallas, TX. Jane L. Forrest is Director, National Center for Dental Hygiene Research & Practice, Ostrow School of Dentistry, University of Southern California, Los Angeles, CA. She represented the American Dental Hygiene Association on the panel. Philippe P. Hujoel is Professor of Periodontics, Department of Oral Health Sciences, School of Dentistry, and Adjunct Professor of Epidemiology, Department of Epidemiology, School of Public Health, University of Washington, Seattle, WA. Kirk W. Noraian is a periodontist in private practice in Bloomington and Urbana, IL. Henry Greenwell is Professor and Director for Graduate Periodontics at the University of Louisville. Julie Frantsve-Hawley was senior director, Center for Evidence-Based Dentistry, Division of Science, American Dental Association, Chicago, IL when this document was written. She is now executive director, American Association of Public Health Dentistry, Springfield, IL. July 2015 Page 2 Cameron Estrich is a health science research analyst, Division of Science, American Dental Association, Chicago, IL. Nicholas (Buck) Hanson is previously a health science research analyst, Division of Science, American Dental Association, Chicago, IL. ACKNOWLEDGMENTS The panel would like to acknowledge the efforts of the following individuals and their commitment in helping complete this project. Dr. Gina Thornton-Evans, a Dental Officer with the Surveillance, Investigations, and Research Team in the Division of Oral Health, Centers for Disease Control and Prevention, Atlanta, GA participated in reviewing evidence throughout the project. Dr. Dave Preble and Dr. Krishna Aravamudhan, ADA Council on Dental Benefit Programs liasions; Dr. Sheila Strock, ADA Council on Access, Prevention, and Interprofessional Relations liaison; Dr. Pam Porembski, ADA Council on Dental Practice liaison; Ms. Malavika Tampi, research assistant, ADA; Ms. Sharon Myaard, senior manager administrative services, ADA; Ms. Kathleen Alexandrakis, senior project assistant; Ms. Kathleen Dennis, senior project assistant. The panel would like to thank the following individuals and organizations whose valuable input during external peer review helped improve this report: Dr. David Sarrett, Virginia Commonwealth University, Richmond, VA; Dr. Robert W. Rives, ADA Council on Dental Benefit Programs nominee, Jackson, MI; Dr. Linda Vidone, Dentaquest/Delta Dental of Massachusettes, Boston, MA: Ashley C. Grill, RDH, BSDH, MPH, ADHA, New York, NY; ADA Council on Dental Practice; Dr. Alpdogan Kantarci, Forsyth Institute and International Academy of Periodontology, Cambridge, MA; Dr. Debora Matthews, Assistant Dean, Research Director Graduate Periodontics Faculty of Dentistry, Dalhousie University, Halifax, Nova Scotia, Canada; Dr. Samantha Rutherford, Scottish Dental Clinical Effectiveness Programme, NHS Education for Scotland, Dundee, Scotland, UK; Professor Helen Worthington, University of Manchester and Cochrane Oral Health Group, Manchester, UK; Dr. Jane C. Atkinson, Center for Clinical Research, NIDCR, Bethesda, MD; Dr. Donald J. DeNucci, Center for Clinical Research, NIDCR, Bethesda, MD; Dr. Sally Hewett, Council on Communications, Bainbridge Island, WA; Dr. Benjamin Youel, Academy of General Dentistry, Chicago, IL; Dr. Jennifer Bone, Academy of General Dentistry, Chicago, IL; and the American Academy of Periodontology, Chicago, IL. Disclosures Dr. Michalowicz has received research support from Ora-Pharma and Atrix Laboratories in the past. Dr. Cobb was the principal investigator of the University of Missouri-Kansas City site for a multicenter clinical trial conducted by OraPharma (Arestin) and has been an unpaid consultant for Hu-Freidy and Livionex. Dr. Hujoel is a national scientific advisor for Delta Dental Plans. Dr. Noraian is a certified instructor for the Institute for Advanced Laser Dentistry. Dr. Greenwell was part of a multicenter study for Millennium Dental Technologies and a laser study for American Dental Technologies. None of the other authors reported any Disclosures. Funding source: Funded by the American Dental Association July 2015 Page 3 Contents 1 Introduction ............................................................................................................................................ 6 2 Methods ................................................................................................................................................. 7 2.1 Panel composition ............................................................................................................................... 7 2.2 Literature search strategy and screening ............................................................................................ 7 2.2.1 Literature search strategy ............................................................................................................. 7 2.2.2. Inclusion/exclusion criteria ........................................................................................................... 7 2.2.3 Definitions ..................................................................................................................................... 9 2.2.4 Grey literature ............................................................................................................................. 11 2.2.5 Electronic database search update ............................................................................................ 11 2.3 Data extraction .................................................................................................................................. 11 2.4 Critical appraisal process – individual studies ................................................................................... 12 2.5 Data synthesis and meta-analysis: Evaluating the effect of the intervention .................................... 12 2.5.1 Choice of outcomes measure, measurement issues, and issues with respect to data adjustments .......................................................................................................................................... 12 2.5.2 Choice of summary effect estimate, meta-analysis model, meta-analysis procedures, and generating forest plots ......................................................................................................................... 13 2.5.3 Sensitivity analyses .................................................................................................................... 14 2.5.4 Statistical heterogeneity .............................................................................................................. 14 2.5.5 Subgroup analysis ...................................................................................................................... 15 2.6 Interpreting CAL results in clinical context ........................................................................................ 15 2.7 Determining the level of certainty in the evidence ............................................................................. 15 2.8 Determining the net benefit rating ..................................................................................................... 18 3 Results ................................................................................................................................................. 18 3.1 Literature search and screening ........................................................................................................ 19 3.1.1 Literature search and screening results ..................................................................................... 19 3.1.2 Characteristics of excluded studies ............................................................................................ 20 3.2 Evidence summary ............................................................................................................................ 21 3.2.1 Scaling and root planing (SRP) ............................................................................................... 25 3.2.2 Systemic sub-antimicrobial dose doxycycline + SRP ............................................................. 29 3.2.3 Systemic antimicrobials + SRP ............................................................................................... 34 3.2.4 Locally-delivered antimicrobials + SRP ................................................................................... 42 3.2.5 Nonsurgical use of lasers + SRP ............................................................................................ 56 3.3 July 2015 Caries data .................................................................................................................................. 69 Page 4 3.4 Smokers versus non-smokers..................................................................................................... 70 4. Discussion ............................................................................................................................................ 70 5. Limitations ............................................................................................................................................ 72 5.1 Of the evidence ........................................................................................................................... 72 5.2 Of the systematic review ............................................................................................................. 73 6. Future research .................................................................................................................................... 74 7. Conclusions ......................................................................................................................................... 75 8. References ........................................................................................................................................... 77 9. Appendices .......................................................................................................................................... 88 Appendix 1 – Literature searches and results ......................................................................................... 88 Appendix 2 – Excluded studies and reasons for exclusion ..................................................................... 92 Appendix 3 – Study characteristics, outcomes data, and critical appraisals of individual trials ............ 124 Appendix 4 – Details on data analysis calculations .............................................................................. 175 Combining results stratified by pocket depth ..................................................................................... 175 Data adjustments for meta-analysis calculations .............................................................................. 175 Specific methods for standard error imputation when data not provided .......................................... 177 July 2015 Page 5 1 Introduction Chronic periodontal disease is a prevalent condition, affecting 47.2% of the adult U.S. population aged 30 and older.1 Chronic periodontal disease results in the loss of toothsupporting connective tissue and alveolar bone and, if untreated, is a major cause of tooth loss in adults. Periodontal disease can be classified as slight, moderate, or severe; the prevalence of moderate has been reported as 30.0% and severe as 8.5%.1 The definition of slight, moderate, and severe for this systematic review is based on Armitage et al.2: Slight disease is defined as 1 or 2 mm of CAL (clinical attachment level) loss, moderate as 3 or 4 mm CAL loss; and severe as greater than or equal to 5 mm CAL loss. There are several therapies available to treat chronic periodontal disease ranging from scaling and root planing (SRP), SRP with adjunctive treatments, to surgical interventions. In 2011, the Council on Scientific Affairs (CSA) of the American Dental Association (ADA) resolved to develop a clinical practice guideline for the nonsurgical treatment by scaling and root planing (SRP) with or without adjuncts on patients with any severity of chronic periodontitis based on an evidence-based systematic review of the literature. In this systematic review, the authors evaluated the effect of SRP alone and in combination with adjuncts on a single clinical outcome of periodontal disease, the change in clinical attachment level. The authors evaluated the following professionally-applied or prescribed medical adjuncts: locally applied antimicrobials (chlorhexidine chips, doxycycline hyclate gel and minocycline microspheres), nonsurgical use of lasers (diode, both photodynamic and non-photodynamic therapies; Nd:YAG; and erbium), systemic antimicrobials, and systemic sub-antimicrobial dose doxycycline. Systemic antimicrobials were categorized separately from systemic sub-antimicrobial dose doxycycline because the mechanisms of action are different. The mechanism of action for subantimicrobial dose doxycycline has been reported as inhibiting mammalian collagenase activity (MMP-8) without a measureable antibiotic effect or development of antibiotic resistance.3, 4 Experimental adjuncts, adjuncts not currently available in the United States, non-prescription (over-the-counter) adjuncts, or surgical treatments were not considered. This systematic review provides the evidence base for the companion clinical practice guideline.5 The authors addressed two main clinical questions and two sub-questions. The main questions are: July 2015 Page 6 1. In patients with chronic periodontitis, does scaling and root planing (SRP) [hand and/or ultrasonic] result in greater improvement of CAL when compared to a) no treatment; b) supragingival scaling and polish (prophylaxis); or c) debridement? 2. In patients with chronic periodontitis, does the use of local antibiotics/antimicrobials, systemic antibiotics, combinations of local and systemic antibiotics, agents for biomodification or host modulation, or nonsurgical lasers with SRP compared to scaling and root planing alone result in greater improvement of CAL? The sub-questions are: a. Is there a change in caries increment among the comparison groups? (note: only use data if papers already included with CAL data) b. Is there a difference between smokers and non-smokers? 2 Methods 2.1 Panel composition The authors comprise a multidisciplinary panel of subject matter experts and ADA staff methodologists convened by the American Dental Association (ADA) Council on Scientific Affairs (CSA). 2.2 Literature search strategy and screening 2.2.1 Literature search strategy The authors developed the search strategy as presented in Appendix 1. Two authors (NBH and CE) used the strategy to search PubMed and Embase. In addition, one author (ST) handsearched references of relevant systematic reviews6-45 to find studies that might have been missed through the electronic sources. 2.2.2. Inclusion/exclusion criteria The authors developed inclusion/exclusion criteria through consensus. The criteria are presented in Table 1. Most of the criteria were developed a priori; however, as typically occurs during systematic reviews, criteria that were not considered a priori are identified during the screening process. To minimize bias that may have affected inclusion/exclusion decisions, the panel experts were presented with de-identified trial specifics by the authors involved in the screening process, and decisions were made on inclusion or exclusion by other authors without knowledge of the trial’s results. July 2015 Page 7 Table 1. Inclusion/Exclusion criteria Inclusion criteria: Human trials Studies with concurrent control Studies with randomized controls – ONLY Randomized or quasi-randomized controlled trials Prospective Published in English language After 1960 At least 6 months in duration Studies that report on chronic adult periodontitis; progressive periodontitis; rapidly progressive periodontitis; recurrent periodontitis; or refractory periodontitis; or periodontitis (unspecified) Split-mouth and parallel-group trials Must report CAL, relative attachment level/loss, probing attachment level/loss or attachment level/loss data in full text to be included Control can have an additional intervention (such as CHX irrigation if treatment arm has the same additional intervention). Saline irrigation is acceptable as part of a control with SRP even if there is no additional intervention. Trials where selected failing pockets are reinstrumented with SRP periodically throughout the study period Medical/prescription adjuncts Exclusion criteria: Non-English Studies that report on aggressive (early onset) periodontitis, aggressive periodontitis, localized aggressive periodontitis, generalized aggressive periodontitis, juvenile periodontitis or “pre-pubertal” periodontitis Studies that do not report CAL or any related attachment level data Anti-inflammatories, statins; other medications with mechanisms of action not related to altering the biological environment; chlorhexidine rinse; experimental interventions; herbals, over-the-counter Studies comparing different SRP delivery methods Treatments (full products, not just active ingredients) that are not commercially available in the United States [for example, Actisite (tetracycline fibers) and Elyzol (metronidazole), chlorhexidine varnish] Trials that apply the first dose of adjunct after 1 week from SRP Trials that reapply adjuncts at selected failing sites during the study (note: this does not apply to studies on SRP alone because of ethical reasons and the standard of care) No exclusions based on length of time that adjunct is administered Although ideally there is no language restriction for systematic reviews, for practical reasons, the panel limited the eligible studies to those available in English. The literature search strategy included searching a primarily European electronic database (Embase) to help offset this limitation by searching for articles of foreign origin. This database provided a source of English language articles published outside the United States that may not be catalogued in Medline. One result of these choices is that literature was retrieved on products available worldwide, but that are not currently commercially available in the United States, such as Elyzol (a topical metronidazole gel). Cognizant of the limitations of split-mouth trials, the panel decided to include both split-mouth and parallel-group trial designs as long as the resulting meta-analyses were presented sub- July 2015 Page 8 grouped by trial design. One limitation to split-mouth trials is that the magnitude and direction of bias are inestimable; however, a recent meta-epidemiological study46 provides some evidence that there is no difference in intervention effect estimates derived from split-mouth and parallelarm RCTs. Another choice the panel made was to include only trials of at least six months in duration. There was concern regarding the volume of studies that would remain with this criterion; however, the panel decided six months was the minimum amount of time that was necessary to observe a lasting therapeutic effect. The panel also noted that this 6-month criterion is used for FDA approval of drugs for treatment or prevention of gingivitis.47 The panel also considered the minimum number of sites measured per participant. It decided to include studies that evaluated at least one site in each patient. The type of probe used to obtain clinical measurements was considered by the panel. Various manual and automated probes, with different incremental markings, were used in different studies. While recognizing that this variability could be a concern, the panel also recognized that skilled and standardized evaluators have been shown to be accurate with many types of manual and automated probes.48 The panel thus elected to use measurements as reported by the authors and to not exclude any type of probing method from consideration. All full text articles were also screened independently and in duplicate by two of the authors (ST, NBH, CE, or JFH) for inclusion. Articles where agreement could not be achieved were presented to the panel for further discussion and a final decision. 2.2.3 Definitions The panel found some variability with definitions in the literature related to periodontal disease, which complicated efforts to screen studies to answer the clinical questions. Others have also commented on the variability of periodontal disease definitions, disease classifications, and measures used to quantify disease.49 The following are definitions that the panel used when conducting this systematic review: Scaling and root planing (SRP): Within this systematic review, “SRP” is defined as noted within the Code on Dental Procedures and Nomenclature (CDT). These codes and their associated nomenclature and descriptors serve to record and report care. SRP should be differentiated from supra- or sub-gingival debridement as noted below from the CDT: July 2015 Page 9 D4341, Periodontal Scaling and Root Planing/per Quad: “Root planing is the definitive procedure designed for the removal of cementum and dentin that is rough and/or permeated by calculus or contaminated with toxins or microorganisms...” D4355, Full mouth debridement: “The gross removal of calculus that interferes with the ability of the dentist to perform a comprehensive oral evaluation. This preliminary procedure does not preclude the need for additional procedures.” Debridement refers to the removal or disruption of dental biofilms and dental calculus from supragingival tooth surfaces and subgingival root surfaces without the deliberate removal of cementum as done in root planing. Thus, it was not considered as an active treatment in this guideline. Some researchers used the term “instrumentation” to describe the procedure performed in their studies; however, this non-specific term was judged to be inconclusive. Similarly, some authors stated that “ultrasonic scaling” or “sub-gingival scaling” was performed. These procedures were often described as “debridement.” The panel decided that treatments that were not described with the specific term “root planing” were excluded. Similarly, if the authors stated they performed “scaling and root planing”, but described the use of ultrasonic instrumentation with no mention of hand instrumentation, the treatment was included. Diagnosis: The clinical questions specifiy a diagnosis of chronic periodontitis for inclusion. After discussion, the panel also included the diagnoses “progressive periodontitis” and “rapidly progressive periodontitis”, although the terms “aggressive periodontitis” and “rapidly aggressive periodontitis” were excluded. The panel wanted to err on the side of inclusion rather than exclusion, and was limited in their confidence that the diagnosis definitions and terminology remained consistent over the years. Disease severity: Many published papers did not define disease severity of the sample population, but if they did, that nomenclature was recorded in the abstraction forms. The panel refers to Armitage2 for standard definitions when severity was not named. Note that the literature included in this review spans a time period wherein the disease severity classification system and nomenclature were changing. The panel decided to include studies on patients with “refractory periodontitis,” although the term is outmoded. July 2015 Page 10 Site: Researchers’ use of the term “site” was often unclear and should be clarified in future reporting. This term was used to indicate a measurement site (e.g. “CAL was measured at six sites per tooth”) as well as a treatment site spanning multiple teeth (e.g. “one site received SRP plus doxycycline hyclate gel”). This interchangability of terminology is particularly troublesome when there is little description of how the mean CAL was calculated for each treatment. The panel evaluated the use of “site” in each paper reviewed and made a determination of the author’s meaning. Attachment level: The clinical question uses the specific term “clinical attachment level.” Some researchers used this term, while other used the terms: “attachment level”, “relative attachment level”, and “probing attachment level.” Some authors specified the exact locations used for the measurement of the attachment level. The panel decided to include all forms of attachment level. 2.2.4 Grey literature The expert panelists were asked whether or not they were aware of any unpublished or nonpeer-reviewed studies that should be considered for inclusion. 2.2.5 Electronic database search update In July 2014, the electronic search strategy was rerun to collect any studies that had been published between the time when the search had been conducted at the start of the project and before completion of the manuscript. The literature was screened by in duplicate by two of three authors (ST, CE, or JF). 2.3 Data extraction A data extraction form was designed using Excel to capture information from each included study with respect to study conduct, participants, disease severity, treatment, adverse events, and CAL outcomes data. A priori rules for data extraction included the preference for 9-month CAL results, and if that time period was not reported but both 6- and 12-month results were reported, the 12-month results were extracted. Two authors extracted data from each included study into the standardized form independently and masked from each other’s work. The two data sets were then compared and adjudicated by a third reviewer [one of four authors (ST, NH, CE, or JF), but different from the original reviewers]. The data from the updated literature search was extracted by one of two authors (ST or JF), and a second author reviewed the information. A summary of the extracted data is July 2015 Page 11 presented in Appendix 3. During a three-day, face-to-face panel meeting, all panelists reviewed and discussed results from each study. 2.4 Critical appraisal process – individual studies The panel critically appraised each included study using Cochrane’s Risk of Bias tool.50 The tool includes six domains for bias assessment: selection, performance, detection, attrition, reporting, and other. The critical appraisal process assesses randomization; allocation concealment; masking of participants, study personnel, and outcomes assessors; the interventions; and the completeness of outcomes data and reporting. The tool requires evaluators to assess whether there is low, unclear, or high risk of bias for that domain item as well as supporting information for each assessment. The tool also provides concrete examples for each domain as to what is considered low, unclear, and high risk methodologies to aid in decision-making. All panel members participated in an orientation webinar to train them on the critical appraisal process. The studies were divided into categories by adjunct type, and two panel members were assigned to each category. Each panel member received between 10-15 studies to review. The panelists entered the appraisals into a standardized form. Independent from the panel members, one of four authors (ST, NH, CE, or JF) duplicated the critical appraisal of all included studies independently and masked from the panel’s review. The two data sets were then compared and adjudicated by a third reviewer [one of four authors (ST, NH, CE, or JF), but different from the original appraisers] to achieve consensus on the Risk of Bias assessment for each domain. The data from the updated literature search was appraised by one of two authors (ST or JF), and a second author reviewed the information. The critical appraisal results are presented in Appendix 3. 2.5 Data synthesis and meta-analysis: Evaluating the effect of the intervention 2.5.1 Choice of outcomes measure, measurement issues, and issues with respect to data adjustments A patient-centered outcome such as tooth loss or quality of life would provide the best evidence on periodontal treatment effectiveness; however, periodontal researchers have mostly reported on surrogate outcomes such as probing depth (PD) and CAL. PD is measured from the gingival margin and the measurement is impacted by gingival recession or inflammation, but CAL is July 2015 Page 12 measured from a fixed reference point (typically the cementoenamel junction) and is a more valid metric and a more stable indicator of improvement in periodontal health. The panel voted to use CAL as the primary outcome to be used on the assessment of periodontal therapies for many reasons including 1) it is used to measure the clinical effect of SRP;36, 51 2) gains in clinical attachment account for roughly 50% of probing depth reduction following SRP of periodontal pockets with 4-6 mm and ≥ 7 mm probing depths;36, 51 3) Imrey et al.52 recommended that CAL or alveolar bone support be used as a primary outcome in non-surgical interventional trials of periodontitis, and they also advocated using CAL as an a priori secondary outcome in trials in which bone loss was the primary outcome; and 4) the Food and Drug Administration (FDA) has generally adopted these recommendations in their product/drug approval process for adjuncts. Regardless of the debate regarding use of CAL vs. PD, the “gold standard” for measuring stability or progression of periodontitis remains CAL.53, 54 Studies reported data in several formats, such as change in mean full-mouth CAL, change at disease/study sites only, or changes stratified by baseline probing pocket depth, to facilitate data aggregation and interpretation. The panel used a set of rules to maximize the uniformity of the data abstraction and analysis processes. The rules include the choice of data presented by disease/study site over that for full mouth or stratified by pocket depth. If the data were only presented stratified by pocket depth, the data were combined as described in Appendix 4. When there was no information on standard deviation or any way to impute from an inferential statistic, the standard deviations of the other studies in its category were averaged55 in most cases, and exceptions are noted in Appendix 4. In some studies, there was one control arm and multiple treatment arms. If these treatments appeared in the same meta-analysis, the number of subjects in the control group was divided by the number of treatment arms for the purposes of the meta-analysis. This adjustment was made to ensure that the control subjects were not over-represented. 2.5.2 Choice of summary effect estimate, meta-analysis model, meta-analysis procedures, and generating forest plots In assessing the efficacy of SRP, the panel compared mean change in CAL between SRP and placebo groups. To assess adjuncts, the panel compared mean changes between groups receiving SRP and those receiving SRP plus the adjunct. The authors used the generic inverse variance method56 as contained in RevMan software57 to calculate the summary effect over all included trials using the random effects model. July 2015 Page 13 The random effects model was chosen because the authors could not assume that the studies were conducted under similar conditions. Although it is possible that the effect size was similar among the studies, the authors assumed the effect size varied among studies because of differences in population age, comorbidities, socio-economic class, and/or clinician experience.58 When interpreting the random effects-generated mean difference and confidence interval, it is important to consider that the “summary effect estimate” is an estimate of a distribution of effects, not a common effect.58 For example, although the 95% confidence interval of the mean effect of a treatment may cross the line of no effect, there is the possibility that the treatment varies by context, such as population age, comorbidities, socio-economic class, or clinician experience.59 2.5.3 Sensitivity analyses Correlation coefficients (designated as ), indicating within-patient correlation in split mouth trials at a given time point or correlation between CAL measures over time, were required for the statistical computations. The initial value of was set at 0.25 following the protocol of a related systematic review60. Since the true value of is unknown, the effect of that assumption on the results of the metaanalysis computations was tested by conducting a sensitivity analysis. The meta-analyses were rerun by changing the value to either -0.1 or 0.9 and observing the effect of the change on the resulting values of the mean difference, 95% confidence interval, and heterogeneity statistics. A similar analysis was conducted removing studies where no information regarding the standard deviation (SD) was reported, which necessitated estimating the SD based on the reported results of similar trials. 2.5.4 Statistical heterogeneity Statistical heterogeneity was interpreted primarily using the tau-squared, p-value of tausquared, and I-squared statistics. The tau-squared statistic was interpreted as an estimate of the between-study variance, the p-value of this statistic was interpreted as the statistical significance of between-study variance, and the I-squared was interpreted as the percent of total variance that is attributable to true between-study variance (as opposed to within-study error variance).58 July 2015 Page 14 2.5.5 Subgroup analysis The authors decided a priori to present the results of the meta-analyses in sub-groups based on trial design. 2.6 Interpreting CAL results in clinical context The panel reviewed the power calculations of included studies to determine the threshold that the authors used to determine statistical significance. These CAL differences ranged from 0.25 mm61 to 1.8 mm62. Caution must be observed with respect to using power calculations as these are not always performed a priori. For the purposes of interpreting the results, the panel made the following clinical relevance scale a priori to reviewing the results (Table 2): Table 2. Clinical relevance scale for interpreting mean differences in CAL CAL Difference Range (mm) 0 - 0.2 >0.2 – 0.4 >0.4 – 0.6 >0.6 Judged clinical relevance Zero benefit Small benefit Moderate benefit Substantial benefit The panel notes that the literature is inconsistent on methodology regarding how many sites and teeth are assessed and analyzed. In particular, some researchers study and analyze only diseased sites, whereas other report whole mouth averages. Whole mouth measurements will tend to underestimate the effect since healthy or less severe sites will be included with more severe sites. Therefore, practitioners are cautioned when comparing average results to individual tooth or tooth site results. 2.7 Determining the level of certainty in the evidence After the data were collected, the authors reviewed the results to determine their level of certainty in the evidence as high, moderate, or low. Several criteria were evaluated to make this determination as follows: 1. The quantity of evidence (number and size of studies) 2. Risk of bias for each outcome (limitations of the evidence across domains and studies) 3. Applicability of evidence 4. Consistency/inconsistency (or unexplained heterogeneity) of results 5. Precision/imprecision (narrow or wide confidence intervals) July 2015 Page 15 6. Probability of publication bias The revised Clinical Practice Guidelines Handbook63 gives more details on evaluating these criteria. During a three-day, face-to-face panel meeting, and at follow up conference calls and through email, all panelists reviewed and discussed results from each study individaully as well as in aggregate on each treatment to arrive at consensus on the level of certainty in the evidence. Table 3 has been developed63 as a tool to aid in capturing the assessments, presenting the assessments in a repeatable manner for each intervention evaluated, and facilitating consistent judgments of evidence criteria to be made between multiple interventions in this systematic review. Table 3. Evidence profile reporting format Therapy aInterpreted Quantity of evidence No. No. Trials participants Level of certainty assessment Risk ConsisAppliPreciof tencya cability sion bias Publication bias Level of Certainty according to the Cochrane Handbook 55 section 9.5.2: The results of the meta-analysis were entered into the evidence profile in the “Mean difference (mm)” column to summarize the treatment effect. The number of studies and participants were also input into the corresponding columns. The panel assessed the risk of bias by making an overall assessment of the critical appraisal data for each domain of every study. The panel judged the applicability of the evidence by assessing whether or not the results could be generalized to a common population. Consistency was evaluated according to the I-squared statistic provided in each meta-analysis according to Table 4.55 Note that when there are less than 20 studies in a meta-analysis, consistency-related statistics have very low power.64, 65 Table 4. Consistency interpretation I-squared 0-40% 30-60% 50-90% 75-100% Interpretation Might not be important May represent moderate heterogeneity* May represent substantial heterogeneity* Considerable heterogeneity* Nomenclature Consistent Moderate inconsistency Substantial inconsistency Inconsistent *The importance of the observed value of I-squared depends on (i) magnitude and direction of effects and (ii) strength of evidence for heterogeneity (e.g. p-value from the chi-square test, or a confidence interval for Isquared). Note that the thresholds overlap and are arbitrary and uncertain66; and also that there is a lot of uncertainty around the I-squared statistic, especially when the number of studies is few (less than three).67 July 2015 Page 16 Mean difference (mm) Precision is judged by the width of the 95% confidence intervals in relation to clinical relevance of the treatment effect. Publication bias is assessed when there are at least 10 studies in an analysis via Egger’s procedures using Stata.68 The panel reviewed all the information of the evidence profile to arrive at a summary level of certainty in the effect estimate as shown in Table 5, which provides a description and types of evidence for each level of certainty. Table 5. Level of Certainty in the body of evidence included within the systematic review. Level of Certainty in Effect Estimate Description High The body of evidence usually includes consistent results from welldesigned, well-conducted studies in representative populations. This conclusion is unlikely to be strongly affected by the results of future studies. This statement is strongly established by the best available evidence. Moderate As more information becomes available, the magnitude or direction of the observed effect could change, and this change could be large enough to alter the conclusion. This statement is based on preliminary determination from the current best available evidence, but confidence in the estimate is constrained by one or more factors, such as: the limited number or size of studies; plausible bias that raises some doubt about the results; inconsistency of findings across individual studies; imprecision in the summary estimate; limited applicability due to the populations of interest; evidence of publication bias; or lack of coherence in the chain of evidence. Low July 2015 More information could allow a reliable estimation of effects on health outcomes. The available evidence is insufficient to support the statement or the statement is based on extrapolation from the best available evidence. Evidence is insufficient or the reliability of estimated effects is limited by factors such as: the limited number or size of studies; plausible bias that seriously weakens confidence in the results; inconsistency of findings across individual studies; imprecision in the summary estimate; gaps in the chain of evidence; Page 17 findings not applicable to the populations of interest; evidence of publication bias; or a lack of information on important health outcomes. 2.8 Determining the net benefit rating This systematic review serves as the foundation for the companion evidence-based clinical practice guideline.5 The methods require a determination of the net benefit rating for the intervention in question. The net benefit rating is assessed by judging the balance between the benefit (estimate of effect from the meta-analysis, which could actually be negative or no benefit) and any potential harms that have been identified by the panel. For each treatment, the panelists selected from one of three options: 1) the benefits clearly outweigh the harms; 2) the benefits and harms are closely balanced, or there is uncertainty in the estimate of the balance; or 3) the harms clearly outweigh the benefits. This determination involves an assessment of several features of the evidence, the details of which are described in the following sections. The panel reviewed the evidence of harms (or adverse events) for each treatment arising from a) the included studies or b) commonly reported adverse events from sources such as the FDA. During a three-day, face-to-face panel meeting, and at follow up conference calls and through email, all panelists reviewed and discussed results from each study individually as well as in aggregate on each treatment to arrive at consensus on the level of certainty in the evidence and the net benefit rating. 3 Results The authors conducted a comprehensive search of the biomedical literature, screened the results of the search according to inclusion/exclusion criteria, critically appraised the included studies, synthesized the data through meta-analyses where appropriate, and evaluated the level of certainty in the evidence regarding the magnitude of the net benefit. A summary of the results of these processes is presented in sections according to treatment. Each section begins with a general description of the studies in terms of number of studies, study design, sample size, country in which the study was conducted, and severity and smoking status of included participants. Note that this information is descriptive, and at this time there were not enough studies with any particular characteristic except study design that would allow a subgroup analysis to be performed based on the characteristic. For example, studies with any severity (slight through severe) of chronic periodontitis were included; however, there was not enough information to differentiate results based on periodontitis severity. July 2015 Page 18 The other subsections are critical appraisal, assessment of publication bias, results of intervention, sensitivity analyses, evidence profile, adverse events assessment (potential for harms), and balancing benefits with potential for harms. Note that translation of this information into a clinical practice guideline is presented in the companion paper5. The supplemental materials (Appendices 1-4) contain further detailed information for the interested reader as follows: Appendix 1- search strategies and results; Appendix 2 - detailed list of excluded studies with reasons; Appendix 3 – study characteristics, outcomes data, and authors’ judgments of risks of bias; and Appendix 4 – details on data analysis calculations. 3.1 Literature search and screening 3.1.1 Literature search and screening results Figure 1 shows the step-by-step results of the literature screening process. PubMed and Embase were searched from 1966 through October 2012 resulting in 1681 records after duplicates were removed. The updated literature search added 315 publications through July 2014, and after duplicates were removed, a total of 1944 records were screened by title and abstract. At least two authors (ST, CE, JF, and NBH) independently screened the titles and abstracts of all the retrieved articles according to the inclusion/exclusion criteria. Any article that either screener selected was retrieved for full text review. There was 77% agreement of full text retrieval by both parties, with the remaining selected by either party individually. Full texts of 440 records were retrieved and reviewed, with the final number of included studies being 72. No citations were found through hand searching that met inclusion criteria. July 2015 Page 19 Figure 1. PRISMA flow diagram of literature search and screening process 3.1.2 Characteristics of excluded studies Appendix 2 lists the excluded studies with reasons for exclusion. The reasons were combined into 12 larger groups to better understand the distribution of reasons for study exclusion. Table 6 lists the exclusion groups and the percentage of the total excluded studies in each group. Note that studies on subgingival irrigation69-77 were identified in the literature search showing July 2015 Page 20 varying effects; however, none of these studies met all the inclusion criteria, and therefore were not eligible to be summarized in this systematic review. Table 6. Categories of reasons for study exclusion after full text review with percentage of studies in each category Percent of excluded Exclusion group Details of studies excluded studies in this category Design Availability Data Publication Duration Adjunct Not RCTs; not randomized; no control; no passive control; head-to-head trials Adjunct not commercially available (compounded by author); adjunct not commercially available in the U.S. for dental applications (e.g. Elyzol, KTP laser, compounded materials, Actisite); experimental therapies (multiple pharmacotherapies) No CAL data; data in unusable format; data not provided separately for treatment Ineligible publication type Less than 6 months in duration Not medical adjunct as defined in inclusion criteria (such as hyperbaric oxygen); therapy not used as adjunct to SRP; insufficient details about use (lasers); experimental therapy Duplicate citation Disease SRP Retreatment Duplicate data Erroneous duplicate citations Aggressive periodontal disease; not chronic periodontal disease; not periodontal disease Not SRP procedure; debridement; SRP methods comparisons; surgical treatment Retreatment at failing sites Data reported in another included publication 23 15 13 11 10 8 6 5 4 3 2 3.2 Evidence summary The panel included 72 randomized clinical trials on nonsurgical treatments for chronic periodontal disease. A summary of the evidence is presented in Table 7. All extracted data are July 2015 Page 21 presented in three tables per each treatment in Appendix 3, one table each for study characteristics, outcomes data, and the authors’ judgment of the risk of bias. July 2015 Page 22 Table 7. EVIDENCE PROFILE SUMMARY TABLE Level of certainty assessment criteria Therapy Quantity of evidence No. No. RCTs participants Risk of bias Consistency Applicability Precision Publication bias Level of Certainty Benefit (Mean difference in CAL) SRP 11 331 Unclear Consistent Yes No serious imprecision None detected p=0.707 Moderate 0.49 (0.36 to 0.62) SRP+systemic subantimicrobi al dose doxycycline 11 813 Unclear Moderate inconsistency Yes No serious imprecision None detected p=0.121 Moderate 0.35 (0.15 to 0.56) Yes No serious imprecision None detected p=0.803 Moderate 0.35 (0.20 to 0.51) SRP+systemic antimicrobials 24 1086 Unclear Substanti al inconsistency SRP+CHX chip 6 316 Unclear Consistent Yes No serious imprecision Too few studies to assess Moderate 0.40 (0.24 to 0.56) SRP+ doxycycline hyclate gel 3 64 Unclear Moderate inconsistency Yes Serious imprecision Too few studies to assess Low 0.64 (0.00 to 1.28) SRP + minocycline microspheres 5 572 Unclear Moderate inconsistency Yes Serious imprecision Too few studies to assess Low 0.24 (-0.06 to 0.55) July 2015 Net benefit rating Moderate benefit outweighs potential for harms Small benefit outweighs potential for harms Balance between small benefit and potential harms Balance between moderate benefit and potential harms Uncertainty in the balance between benefits and harms because benefits are unclear Uncertainty in the balance between benefits and harms because Page 23 SRP + diode laser (PDT) SRP + diode laser (nonPDT) 10 4 306 98 Low Inconsistent Yes Serious imprecision None detected (p=0.679) Moderate 0.53 (0.06 to 1.00) Unclear Substanti al inconsistency Yes Serious imprecision Too few studies to assess Low 0.21 (-0.23 to 0.64) No evidence of a benefit Yes Serious imprecision Too few studies to assess; expert knowledge of unpublished studies Low 0.41 (-0.12 to 0.94) No evidence of a benefit Yes Serious imprecision Too few studies to assess Low 0.18 (-0.63 to 0.98) No evidence of a benefit SRP + Nd:YAG laser 3 82 Unclear Moderate inconsistency SRP+erbium lasers 3 82 Low Inconsistent July 2015 benefits are unclear Uncertainty in magnitude of moderate benefit balanced with potential harms Page 24 3.2.1 Scaling and root planing (SRP) General description of studies Eleven studies met inclusion criteria reporting the effect of SRP compared to no treatment, supragingival scaling, or debridement on chronic periodontitis.78-87 Six were split-mouth78-83 and five were parallel-group84-88 trial designs. All studied small sample sizes (from 7 to 43 per group). The studies were conducted worldwide, including Sweden, China, Germany, Italy, Brazil and the U.S. from 1983 through 2014. The severity of periodontal disease was described as moderate to advanced (severe) in five studies79, 81, 83, 85, 87, advanced (severe) in three studies78, 80, 86, and not stated in two studies82, but the probing pocket depth of included participants was described as ≥ 5 mm in one study and attachment loss > 3 mm in another88. One study84 was exclusively on participants with Type 2 diabetes, and another88 exclusively on participants with chronic obstructive pulmonary disease. One86 of the studies excluded smokers, three79, 82, 83 studies included smokers, but did not perform a sub-group analysis reporting results by smoking status, and seven78, 80, 81, 84, 85, 87, 88 studies did not mention the smoking status of the participants. Critical appraisal Details for each domain and the support for each judgment for each individual study are presented in Appendix 3. Slightly less than half the study arms were at a low risk of bias for random sequence generation, one was inadequate, and the rest were unclear as to how randomization was performed. Four studies reported an adequate concealment procedure for allocation, two were inadequate, and the rest were unclear as to how allocation was concealed. Only three studies masked participants, and two masked personnel. The panel noted that SRP masking from either the participants or personnel would be extremely difficult, and did not weigh this dimension highly. Most (73%) studies treated the groups the same except for the intervention. Most studies (64%) masked the outcomes assessor and reported complete outcomes data. There was no indication of selective reporting in 91% of the studies. The judgments of high, unclear, or low risk for bias as a percentage of included studies in this section are depicted by domain in Figure 2. The main issues for bias for this group of studies were lack of adequate randomization procedures, allocation, and masking of personnel and participants. The panel judged the overall risk of bias of the included studies as unclear. July 2015 Page 25 Figure 2. Review authors’ judgment of each risk of bias item as low, unclear, or high as a percent of included studies Assessment of publication bias Since there were over 10 studies included comparing SRP to no treatment, an assessment of publication bias by visual inspection of the funnel plot as well as Egger’s test were undertaken. Figure 3 shows the funnel plot with 11 studies and 13 arms. The panel found no evidence of publication bias (Egger’s test result, p=0.707). .2 .4 .6 SE Mean Difference 0 Funnel plot with pseudo 95% confidence limits -1 0 1 2 Mean Difference Figure 3. Funnel plot of studies on SRP July 2015 Page 26 Results of intervention Figure 4 shows the meta-analysis sub-grouped by study design comparing SRP to no treatment. The overall group mean difference is 0.49 mm gain in clinical attachment level (i.e., improvement) with SRP treatment versus no treatment with a 95% CI from 0.36 to 0.62 mm. Figure 4. Meta-analysis of studies on SRP sub-grouped by study design; mean difference in units of millimeters. Notes: Lindhe, used Kahl data for SE calculations, see Appendix for details; Neill, data in figure and text inconsistent, used figure data; Ng, used final data since change not reported; Berglundh, used Kahl data for SE calculations, see Appendix for details; Kahl, see details in Appendix for calculations; Rotundo, used final data since change not reported; Jones, Note that very small SE impacts the weight; however, the data were extracted from the paper figure without adjustment; Van Dyke, used all treated site change data although data available stratified by pocket depth; converted SE to SD for calculations; Riberio, combined change data stratified by pocket depth; Chen, calculated change from baseline and final data; divided control "n" by 2 because two intervention arms; Zhou, calculated change from baseline and final data; divided control "n" by 2 because two intervention arms. Sensitivity analyses Because assumptions were made with respect to choosing a correlation coefficient for the statistical calculations, the sensitivity of the result to these assumptions was investigated. Table 8 shows the impact of changing the correlation coefficient from the initial value of 0.25 to both 0.1 and 0.9. At correlation coefficients of -0.1, the mean difference did not change; however, at the correlation coefficient of 0.9, the mean difference increased by 0.10 mm. The heterogeneity did not change at -0.1, and it increased substantially at 0.9. In three studies, problems were July 2015 Page 27 identified with study methodology78-80, so the SEs were imputed (see Appendix for methods). The results without these studies are shown in the third row of the table. Results without Jones are shown in the final row. Table 8. Summary of sensitivity analyses using the random effects model. Treatment SRP SRP Without study with imputed standard errors† Without Jones † Hetero- Mean geneity p- difference (%) value (mm) 0 0.08 0 75 0.86 <0.00001 0.49 0.59 0.35, 0.62 0.40, 0.78 8 0 0 0.92 0.47 0.33, 0.60 10 0 0 0.62 0.48 0.24, 0.71 Correlation # of Hetero- coefficient studies geneity 2 -0.1 0.9 11 11 0.25 0.25 Heterogeneity I2 95% CI Lindhe 1983, Berglundh 1998, Kahl 2007 Evidence profile The summary of findings is summarized in Table 9. The panel judged the overall level of certainty in the evidence to be moderate. Although there were 10 studies, the number of participants was small. The panel reminds readers that the studies included in this analysis randomized patients to receive SRP or no treatment. There are many other clinical studies that did not meet inclusion criteria that use SRP as the positive control treatment. Table 9. Evidence profile for SRP treatment versus no treatment. Level of certainty assessment Therapy SRP vs. no treatment Quantity of evidence No. No. RCTs participants 11 331 Risk of bias Consistency Applicability Precision Publication bias Unclear Consistent Yes No serious imprecision None detected p=0.707 Level of Certainty Moderate *Interpreted as 0.49 mm mean gain in clinical attachment level with SRP treatment, with a 95% CI from 0.36 mm to 0.62 mm improvement. Adverse events assessment (potential for harms) Any type of root planing, including hand and ultrasonic instrumentation, carries the risk of damaging the root surface and potentially causing tooth/root sensitivity. Generally expected post-SRP procedural adverse events include discomfort. One study83 comparing SRP to supragingival debridement (with two other treatments including lasers that are not described here) measured patient-reported pain, dental hypersensitivity, and chewing discomfort according to a visual-analog scale. At the end of treatment, there was a July 2015 Page 28 Mean difference in CAL (mm) 0.49 higher (0.36 to 0.62)* statistically significant difference in pain (higher for SRP than supragingival debridement). After one week, the only statistically significant difference was found in dental hypersensitivity, which was higher with SRP than supragingival debridement. There were no statistically significant differences in the three patient-reported indices at 3 or 6 months after treatment. Another study86 also compared SRP to supragingival debridement, and reported no statistically significant differences in pain, number of analgesics, fever, or oral ulceration the day of or the next day after treatment. Two studies81, 84 reported that no adverse events (AEs) occurred, and five78-80, 82, 85 did not solicit information or report on AEs. There is a report of an increase in cytokine levels.89 Overall, the panel judged the potential for harms from SRP to be negligible. Balancing benefits with potential for harms The panel judged that the moderate benefit of SRP outweighed the potential for harms. 3.2.2 Systemic sub-antimicrobial dose doxycycline + SRP General description of studies Sub-antimicrobial dose doxycycline (SDD, Periostat®) treats periodontal disease by a mechanism called host modulation, which refers to the concept of modulating the host’s response to the presence of bacteria with methods such as inhibiting collagen-destructive enzymes.42, 90 Eleven studies61, 62, 91-100 (in 12 publications) met inclusion criteria reporting the effect of SRP plus LDD versus SRP alone. All were parallel-group trials. There was a varying range of sample sizes from 7 to 133 per treatment group. The studies were conducted worldwide including four in the U.S., four in Turkey (three from one author), and one each in the U.K., India, and Saudi Arabia. The studies were published between 2000 and 2011. With respect to populations, one study included only geriatric participants62 and two only diabetics91, 93. The severity of periodontal disease was described as moderate-to-severe in five of the studies61, 62, 94-97, severe in one study98, and not stated in five studies91-93, 99, 100, but in three studies the probing pocket depth of included participants was described as > 4 mm99 or ≥ 5 mm but less than or equal to 891 or 992 mm. Of the sub-antimicrobial dose doxycycline studies, no other special populations were noted except smoking status. One study100 included only smokers, seven61, 62, 92, 94-98 included smokers but did not perform a sub-group analysis, and three91, 93, 99 did not describe the smoking status of their participants. July 2015 Page 29 Studies on other host modulators considered to be experimental were excluded from this section: a study101 on another form of SDD that is administered as a single 40 mg dose (Oracea®); two studies on bisphosphonates102, 103; and three on other anti-inflammatory drugs104-106. Critical appraisal Details for each domain and the support for each judgment for each individual study are presented in Appendix 3. Eighty-two percent of the studies were at a low risk of bias for random sequence generation. Only four studies reported an adequate concealment procedure for allocation while the other studies did not provide a description. Most of the studies masked the participants and personnel. All studies treated groups the same except for the intervention. Twothirds of the studies masked the outcomes assessor. Just under half had complete outcomes data and were judged to be at low risk for attrition bias, while the same number had significant loss of participants (over 20%) and were judged to be at high risk of attrition bias. No evidence of selective reporting was found. The judgments of high, unclear, or low risk for bias as a percentage of included studies in this section are depicted by domain in Figure 5. The main issue for bias for this group of studies was incomplete outcomes data and the risk for attrition bias. The panel judged the overall risk of bias of the included studies as unclear. Figure 5. Review authors’ judgment of each risk of bias item as low, unclear, or high as a percent of included studies. July 2015 Page 30 Assessment of publication bias Since there were over 10 studies included comparing systemic sub-antimicrobial dose doxycycline plus SRP to SRP alone, an assessment of publication bias by visual inspection of the funnel plot as well as Egger’s test were undertaken. Figure 6 shows the funnel plot with the studies identified by their sub-group. The panel found no evidence for publication bias (Egger’s test, p=0.121). Figure 6. Funnel plot of studies on SRP plus SDD versus SRP alone. .4 .6 1 .8 SE Mean Difference .2 0 Funnel plot with pseudo 95% confidence limits -2 -1 0 1 Mean Difference 2 3 Results of intervention Figure 7 shows the meta-analysis for the included studies comparing SRP to SRP plus SDD. The overall group mean difference is 0.35 mm mean gain in clinical attachment level when SDD was used adjunctively with SRP versus SRP alone with a 95% CI from 0.15 to 0.56 mm improvement. One study62 on a population of geriatric participants appears to be primarily responsible for the observed heterogeneity in these results. July 2015 Page 31 Figure 7. Meta-analysis of studies on SRP plus SDD versus SRP alone; mean difference in units of millimeters. Notes: Caton, Mohammad, Preshaw, Combined change score data that were stratified by disease severity (pocket depth) and converted SE to SD for calculation; Emingil (all three citations, 2004, 2008, 2011) and Gurkan, Study site data used in preference to whole mouth or pocket-depth-stratified data; change scores calculated; Haffajee, Change score provided; SE converted to SD; data estimated from figure; Needleman, ANCOVA results; 95% CI 0.64, 0.18; Al Mubarak, Calculated change from baseline and final data; converted SE to SD; data from experimental sites only; Deo, Error in manuscript and stated change score SD judged to be SE therefore converted;. Sensitivity analyses Because assumptions were made with respect to choosing a correlation coefficient for the statistical calculations, the sensitivity of the result to these assumptions was investigated. Table 10 shows the impact of changing the correlation coefficient from the initial value of 0.25 to both 0.1 and 0.9. At both correlation coefficients -0.1 and 0.9, the mean difference and 95% CI did not change appreciably from those found at the initial value of 0.25. At all correlation coefficients, there is a very small amount of true between-study variation. The summary estimate is judged not to be sensitive to the assumptions that were made. Table 10. Summary of sensitivity analyses using the random effects model. Adjunctive Treatment Correlation coefficient N of studies Heterogeneity 2 Heterogeneity I2 (%) Heterogeneity P-value SRP+SDD SRP+SDD -0.1 0.9 11 11 0.04 0.04 43 51 0.06 0.03 July 2015 Mean difference (mm) 0.35 0.36 Page 32 95% CI 0.15, 0.56 0.18, 0.54 Evidence profile The evidence profile is summarized in Table 11. The panel judged the overall level of certainty in the evidence to be moderate based on the quantity of evidence, the overall risk of bias, the consistency, applicability, and no evidence of publication bias. Table 11. Evidence profile for SRP+systemic low dose doxycycline versus SRP alone. Level of certainty assessment Therapy Quantity of evidence No. No. RCTs participants Risk of bias Consistency Applicability Precision Publication bias Level of Certainty None detected Moderate p=0.121 *Interpreted as 0.35 mm mean gain in clinical attachment level with systemic LDD adjunctive use with SRP versus SRP alone, with SRP + SDD vs. SRP 11 813 Unclear Moderate inconsistency Yes No serious imprecision a 95% CI from 0.15 mm to 0.56 mm improvement. Adverse events assessment (potential for harms) The studies reported that SDD was well tolerated92, 94-97 with no participants reporting AEs93-97 or that the incidence of AEs was similar between the SDD and placebo groups92, 107. The AEs that were judged to possibly be related to SDD were dizziness92, 99 and tachycardia99. In one study, AEs were only reported in the placebo group100. Three studies did not report on AEs62, 91, 98. The FDA label108 lists the following warnings for Periostat® (this is a partial list, and includes warnings for the tetracycline class of drugs): May cause permanent discoloration of the teeth during tooth development; enamel hypoplasia reported; special consideration should be given to pregnant women; may cause an increase in BUN; photosensitivity manifested by sunburn reaction has been observed, and patients are advised to avoid exposure to direct sunlight or ultraviolet light. The FDA label108 lists special precautions such as overgrowth of nonsusceptible microorganisms including fungi, and caution is advised for those with a history of predisposition to oral candidiasis. The FDA label108 lists the most frequent adverse reactions that occurred during clinical trials as headache, common cold, flu symptoms, and toothache. Other adverse reactions to tetracyclines are listed. The panel judged that antimicrobial resistance should not be a factor at sub-antimicrobial dose. Overall, the panel judged the potential for harms from SDD was negligible. July 2015 Page 33 Mean difference in CAL (mm) 0.35 higher (0.15, 0.56)* Balancing benefits with potential for harms The panel judged the small benefit outweighs the potential for harms. 3.2.3 Systemic antimicrobials + SRP General description of studies Twenty-four studies78, 80, 82, 99, 109-128 met inclusion criteria reporting the effect of SRP plus a systemic antimicrobial versus SRP alone. All were parallel-group trials. The sample sizes were relatively small, ranging from 7 to 46 per treatment group. The studies were conducted worldwide including three in the U.S.; three in Sweden and Brazil; two each in Switzerland, Germany, , India, and Japan; one each in Canada, England, Greece, Norway, Spain, and Turkey; and one in both the U.S. and Sweden. The studies were published between 1983 and 2014. The panel decided to combine all antimicrobials into one treatment class for an overall analysis and one evidence profile. The studies reported on five major groups of antimicrobials: amoxicillin/metronidazole combination therapy78, 110, 111, 113, 121, 126, metronidazole99, 118, 127, erythromycin analogues (azithromycin112, 115, 117, 122-125 and clarithromycin119), moxifloxacin114 (a fourth generation fluoroquinolone antibacterial agent), and others, e.g., tetracycline80, 109, 120 and doxycycline128 82, 114 (antimicrobial dose of doxycycline, not to be confused with sub-antimicrobial dose, which is covered in a separate section). One of the studies110 described the severity of the disease of included participants as slight, one117 included only participants with moderate disease, seven113, 115, 116, 118, 119, 125, 128 included participants with moderate-to-severe (or advanced) disease, nine78, 80, 109, 112, 114, 120-122 included participants with severe/advanced disease, and six did not state the severity of disease, but described the probing pocket depth as ≥4 mm99, 123, ≥5 mm82, 126, 127, or ≥ 6 mm111. Two studies included only diabetics.126, 128 Seven112, 119, 121, 123-125, 128 of the studies excluded smokers, eight82, 99, 110, 113, 114, 116, 117, 120 included smokers but did not perform a sub-group analysis reporting the results by smoking status, six78, 80, 109, 111, 126, 127 studies did not mention the smoking status of the participants, one115 study included only smokers, and one118 study reported results sub-grouped by smoking status. There was a variety of dosing regimens for each systemic antimicrobial drug. Table 12 lists the dosing regimens by drug and study citation. July 2015 Page 34 Table 12. Systemic antimicrobial dosing regimens used in research studies Drug Dose Timing Author/citation AMX/MET 375 mg AMX+250 mg MET tid for 14 days Berglundh 1998 AMX/MET 375 mg AMX+500 mg MET tid for 7 days Cionca 2009 AMX/MET 375 mg AMX+250 mg MET tid for 8 days Flemmig 1998 AMX/MET 500 mg AMX bid + 250 mg MET tid for 14 days Goodson 2012 AMX/MET 500 mg AMX+400 mg MET tid for 14 days Miranda 2014 AMX/MET 375 mg AMX+ 250 mg MET tid for 7 days Mombelli 2005, Ribeiro 2009 Metronidazole 250 mg tid for 14 days Haffajee 2007 Metronidazole 200 mg tid for 7 days Palmer 1998, 1999 Metronidazole 400 mg tid for 10 days Preus 2013 Azithromycin Dose not stated qd 3 days prior to SRP Gomi 2007 Azithromycin 500 mg qd for 3 days Haffajee 2007, Oteo 2010 Azithromycin 500 mg qd for 3 days Han 2012 Azithromycin 500 mg qd for 3 days Martande 2014 Azithromycin 250 mg bid first day / 250 mg qd following 4 days Mascarenhas 2005 Azithromycin 500 mg qd 5 days Sampaio 2011 Azithromycin 500 mg qd 3 days prior to SRP Yashima 2009 Clarithromycin 500 mg bid for 3 days Pradeep 2011 Moxifloxacin 400 mg qd for 7 days Guentsch 2008 Tetracycline 250 mg Every 6 hours for 14 days Al-Joburi 1989 Tetracycline Tetracycline 250 mg qid for 2 weeks / 250 mg qd for 48 weeks 250 mg qid for 3 weeks Lindhe 1983 Ramberg 2001 Doxycycline 200 mg first day / 100 mg per day for 9 days Guentsch 2008 Doxycycline 200 mg first day / 100 mg per day for 6 weeks Ng 1998 Doxycycline 200 mg loading dose, then 100 mg qd for 20 days Tsalikis 2014 Qd=once per day; bid=twice per day; tid=three times per day; qid=four times per day Critical appraisal Details for each domain and the support for each judgment for each individual study are presented in Appendix 3. Over half the studies were at a low risk of bias for random sequence July 2015 Page 35 generation. Only six studies reported an adequate concealment procedure for allocation, one reported a procedure at high risk of bias, while the other studies did not provide a description. Half the studies did not mask the participants and personnel. Most studies (80%) treated groups the same except for the intervention. Seventy percent masked the outcomes assessor. Almost 60% had complete outcomes data, and 67% were judged to be at low risk for selective reporting bias. The judgments of high, unclear, or low risk for bias as a percentage of included studies in this section are depicted by domain in Figure 8. The main issues for bias for this group of studies were lack of masking of personnel and participants and unclear allocation concealment. The panel judged the overall risk of bias of the included studies as unclear. Figure 8. Review authors’ judgment of each risk of bias item as low, unclear, or high as a percent of included studies. Assessment of publication bias Since there were over 10 studies included comparing systemic antimicrobials plus SRP to SRP alone, an assessment of publication bias by visual inspection of the funnel plot as well as Egger’s test were undertaken. Figure 9 shows the funnel plot with the studies identified by their sub-group. The panel found no evidence for publication bias (Egger’s test, p=0.803). July 2015 Page 36 Figure 9. Funnel plot of studies on SRP plus systemic antimicrobials. 1.5 1 Test SD .5 0 Funnel plot with pseudo 95% confidence limits -4 -2 0 Test Mean 2 4 Results of intervention Figure 10 shows the meta-analysis for the included studies comparing SRP to SRP plus systemic antimicrobials sub-grouped by systemic antimicrobial type. The overall group mean difference is 0.35 mm mean gain in clinical attachment level when systemic antimicrobials were used adjunctively with SRP versus SRP alone with a 95% CI from 0.20 to 0.51 mm improvement. July 2015 Page 37 Figure 10. Meta-analysis of studies on SRP plus systemic antimicrobials versus SRP alone, subgrouped by antimicrobial type; mean difference in units of millimeters. Notes: 1.1.1 - Berglundh, see appendix for SE calculations; Flemmig, stratified site data combined; SE averaged from other studies in the subgroup; Mombelli, see appendix for MD and SE calculations; Riberio, stratified site data combined for mean and SD of change score; Cionca, no adjustments necessary; Goodson, SE converted to SD to calculate SE of difference in change July 2015 Page 38 score; Miranda, Stratified site data combined for mean and SD of change score; 1.1.2 - Palmer 1999, data are identical to Palmer 1998 where the population was combined; Haffajee, use change data, but figure SE unclear, but estimated at 0.1 mm then calculated SE of difference; divide control group n by 2 since 2 test arms; used all site data although results for PD> 6 mm available; Preus, Change score provided; converted 95% CI to SD; 1.1.3 - Mascarenhas, used all sites data although data stratified by PD are available; although error data stated SE, it was judged to be SD; change score calculated with time correlation coefficient; Gomi 2007, change score means calculated from baseline and final results; Haffajee, see section 1.1.2; Yashima, used change score data; divided control n by 2 since two test arms; calculated SE from SD of change score and n; Oteo 2010, converted SE to SD to calculate difference SE; Sampaio, used full mouth change scores although stratified data available; Han, No adjustments necessary; Martande, No adjustments necessary; 1.1.4 - Pradeep, change scores used at 9 months; 1.1.5 - Guentsch, used change score data and calculated SE from SD in paper and divided control n by 2 since 2 treatment arms; 1.1.6 - Lindhe, see appendix for SE calculations; Al-Joburi, calculated combined score from stratified data for baseline and final data, then used time correlation coefficient for difference calculations; Ng, see appendix for SE calculations; Ramberg, used change score data and calculated SE from SD in paper; number of participants at 1 year not reported so used conservative estimate of number at 13 years; Guentsch, see 1.1.5; Tsalikis, Used change score data and calculated SE from SD in paper. Sensitivity analyses Because assumptions were made with respect to choosing a correlation coefficient for the statistical calculations, the sensitivity of the result to these assumptions was investigated. Table 13 shows the impact of changing the correlation coefficient from the initial value of 0.25 to both 0.1 and 0.9. At both correlation coefficients -0.1 and 0.9, the mean difference and 95% CI did not change appreciably from those found at the initial value of 0.25. At all correlation coefficients, there is a small amount of variation, the majority of which is due to true betweenstudy variation. Three studies in the AMX/MET sub-group and two studies in the tetracycline sub-group did not report standard deviations or standard errors, or there were concerns with the reported standard deviations, so standard errors were estimated. The third row of the table (Systemic antimicrobials, without studies with estimated standard errors) shows the mean difference and 95% CI with those studies excluded from the calculation; the results of which indicate that their inclusion did not substantially impact the summary estimates of mean difference. The fourth row (AMX/MET sub-group, without studies with estimated standard errors) shows the effect of eliminating three studies from AMX/MET sub-group on the results. The mean difference has increased from 0.39 to 0.46, and the 95% CI widens and the result becomes less precise. One study119 on clarithromycin appears to be primarily responsible for the heterogeneity in the results and the presence of sub-group differences. When this study is removed from the metaanalysis, the mean difference decreases and the overall heterogeneity decreases. July 2015 Page 39 Table 13. Summary of sensitivity analyses using the random effects model. Adjunctive Treatment Systemic antimicrobials Systemic antimicrobials Systemic antimicrobials, without studies with estimated standard errors† AMX/MET subgroup, without studies with estimated standard errors†† Without clarithromycin (Pradeep) Correlation coefficient # of studies Heterogeneity 2 Heterogeneity I2 (%) Heterogeneity p-value Mean difference (mm) -0.1 24 0.11 75 <0.00001 0.35 0.19, 0.50 0.9 24 0.14 82 <0.00001 0.39 0.23, 0.55 0.25 19 0.11 78 <0.00001 0.33 0.16, 0.49 0.25 3 0.25 83 0.0004 0.46 -0.09, 1.01 0.25 23 0.04 51 0.001 0.29 0.17, 0.41 95% CI † Ng 2008, Berglundh 1998, Flemmig 1998, Lindhe 1983, and Mombelli 2005 †† Berglundh 1998, Flemmig 1998, and Mombelli 2005 Evidence profile The evidence profile is summarized in Table 14. The panel judged the overall level of certainty in the evidence to be moderate based on the quantity of evidence, the overall risk of bias, the consistency, applicability, and lack of evidence of publication bias. Table 14. Evidence profile for SRP+systemic antimicrobials versus SRP alone. Level of certainty assessment Therapy Quantity of evidence No. No. RCTs participants Risk of bias Consistency Applicability Precision Publication bias Level of Certainty SRP+systemic None Substantial No serious antimicrobials 24 1086 Unclear Yes detected Moderate inconsistency imprecision vs. SRP alone p=0.803 *Interpreted as 0.35 mm mean gain in clinical attachment level with SRP plus systemic antimicrobial adjunctive use versus SRP alone, with a 95% CI from 0.20 mm to 0.51 mm improvement. Adverse events assessment (potential for harms) In the amoxicillin/metronidazole sub-group of studies, gastrointestinal problems such as diarrhea and cramps were observed more often in the test group than the placebo group110, 111, 126 ; as well as nausea/vomiting, headache, and metallic taste126; however, there were no statistically significant differences compared to placebo126. One study113 reported no serious July 2015 Page 40 Mean difference in CAL (mm) 0.35 higher (0.20 to 0.51)* adverse events associated with any of the treatments, but one participant dropped out of the test group because of nausea and vomiting and another developed candidiasis. One study121 reported no acute problems such as periodontal abscesses during the study period. The study121 also reported nausea and diarrhea in four test and three control participants. Two studies78, 116 did not report on adverse events. In the metronidazole sub-group of studies, two adverse events were reported in one study99 in the test group: dizziness and diarrhea. One study reported diarrhea, nausea/headache, feeling unwell, metallic taste, and dizziness, occurring more often with metronidazole, but the differences compared to placebo were not statistically significant127. The other study118 did not report on adverse events. In the azithromycin sub-group of studies, these events were possibly associated with the use of the drug: diarrhea112, 117, 122, 123, allergic reaction99, and difficulty swallowing the tablets99. One study124 reported no adverse events, and two studies115, 125 did not report on adverse events. With respect to clarithromycin, no adverse reactions or discomfort were reported by the test group, except for unpalatable taste and mild gastric intolerance.119 With respect to moxifloxacin, no adverse events were reported.114 However, the FDA issued a box warning for this drug as being associated with increased risk of tendinitis and tendon rupture, exacerbation of muscle weakness in persons with myasthenia gravis and associated breathing problems. With respect to tetracycline, one study reported a case of severe diarrhea109. Two studies did not report on adverse events.80, 120 For doxycycline, one study reported no adverse events114 and the other two did not report on adverse events.82, 128 Antimicrobials as a class of drugs are well known to cause allergic reactions in some people. Other adverse effects are commonly reported (this list is not exhaustive) such as rash, diarrhea, abdominal pain, and/or nausea/vomiting. Additionally, the overuse of antimicrobials promotes the development of resistant strains of bacteria, which are a risk to the population. In general, this class of drugs should be reserved for short-term (less than 21 days) use only, although lower doses may be acceptable over a longer time period. July 2015 Page 41 Lastly, if antimicrobials are overused or misused, there is the risk of the development of antimicrobial-resistant bacteria.129 Balancing benefits with potential for harms The panel judged the small benefit is likely to be balanced with the potential for harms. 3.2.4 Locally-delivered antimicrobials + SRP Three therapies are included in this section on locally-delivered antimicrobials as adjuncts to SRP: chlorhexidine (CHX) chips, doxycycline hyclate gel, and minocycline microspheres. Several studies were excluded from this section because the local antimicrobial products were used as stand-alone applications and not as adjuncts to SRP.130-132 Another category causing exclusion is lack of commercial availability of the product in the U.S., such as tetracycline fibers (“Actisite”) or strips133-143, minocycline gel144-147, metronidazole gel (“Elyzol”)148-155, clarithromycin gel156, 157, azithromycin gel158, CHX varnish,159-161 CHX gel,162-166 and other researcher-prepared gels such as EDTA gel167 and chitosan gel168. 3.2.4.1 CHX chip + SRP General description of studies Six studies compared the effects of SRP plus the local delivery of chlorhexidine chips to SRP alone on chronic periodontitis.169-174 Four were split-mouth169, 171-173 and two were parallelgroup170, 175 trial designs. All but two172, 173 had small sample sizes (ranging from 12 to 25 participants per group); the larger studies included between 82 and 116 participants per treatment arm. The studies were conducted in Turkey, Greece, the U.K., Italy, and Germany from 2001 through 2011. All studies included participants with moderate-to-severe (or advanced) chronic periodontitis as described by the authors or assessed from their inclusion criteria. Four studies170-173 excluded smokers (one170 excluded “heavy” smokers, defined as smoking more than 10 cigarettes per day), one175 included smokers but did not separate the results by smoking status, and one169 did not report smoking status. Because of the variety of smoking inclusion/exclusion criteria, we were unable to determine if the treatment effects differed in smokers and non-smokers. The extracted data are presented in two tables in Appendix 3, one table each for study characteristics and CAL data. July 2015 Page 42 Several studies were excluded from this category for the following reasons. In several CHX chip studies (Grisi et al.176, Jeffcoat et al.177, Jeffcoat et al.178, Mizrak et al.179, Soskolne et al.180) sites with CAL greater than or equal to 5 mm that remained after the baseline chip placement were retreated with new CHX chips. The panel excluded these studies from the analysis because the control subjects with failing sites were not retreated in any manner, leading to a design bias in favor of the CHX chip. Another study (Rodrigues et al. 181) placed the chip three months after SRP, which the panel judged to be part of maintenance therapy and not adjunctive to SRP. Critical appraisal Details for each domain are presented in Appendix 3. Half the studies reported an adequate method of randomization, while half did not describe the randomization method. No studies reported methods for allocation concealment. In all studies, either the participant or the clinician was unmasked or their masking was unclear. In all studies but one173, the groups were treated the same except for the intervention. The outcomes assessor was masked in five studies, but in one169 the masking was unclear. Three of the studies had less than 10% drop-outs, one had 11%, and two did not report this information. There was some indication of selective reporting in two studies172, 173 where mistakes in reporting were noted as omissions in data reporting. The judgments of high, unclear, or low risk for bias in this section are depicted by domain in Figure 11. Because many domains of the included studies were judged to have unclear and/or high risk of bias, the panel judged the risk of bias from this body of evidence as unclear. July 2015 Page 43 Figure 11. Review authors’ judgment of each risk of bias item as low, unclear, or high as a percent of included studies. Results of intervention Figure 12 shows the meta-analysis for the included studies comparing SRP to SRP plus chlorhexidine chips sub-grouped by study design. The overall group mean difference is 0.40 mm mean gain (95% CI: 0.24 to 0.56 mm) in clinical attachment level (i.e., improvement) when chlorhexidine chips were used adjunctively with SRP treatment versus SRP alone. There was no statistically significant difference between the group means according to study design. July 2015 Page 44 Figure 12. Meta-analysis of studies on SRP plus CHX chip versus SRP alone, sub-grouped by study design; mean difference in units of millimeters. Notes: Heasman, CAL change score reported and used; 2002, CAL change score provided in graphical form (endpoint data not reported); converted SE to SD; Azmak, CAL change score provided in graphical form (endpoint data not reported); converted SE to SD; Paolantonio (A), "all site" RAL change score estimated from Figure 4a in publication and converted SE to SD; Paolantonio (B), final data used; Dimitra, CAL change score calculated; Gonzales, mean CAL gain reported but no SD, averaged SDs from 4 other studies to impute the SD. The split-mouth trials showed a statistically significant effect with chlorhexidine chip use, whereas the parallel-group trials did not; however, subgroup analysis indicated no difference between the subgroups. Sensitivity analyses Because assumptions were made with respect to choosing a correlation coefficient for the statistical calculations, the sensitivity of the result to these assumptions was investigated. Table 15 shows the impact of changing the correlation coefficient from the initial value of 0.25 to both 0.1 and 0.9. At a correlation coefficient of -0.1, neither the mean difference nor the heterogeneity statistics changed substantially. At a correlation coefficient of 0.9, the mean difference remains statistically significant favoring adjunctive CHX chip use, but the magnitude of the mean difference has decreased. The amount of between study variation is small and a large proportion of it is true and not due to random error. One study170 did not report an SD, so it was imputed by averaging the SDs from the other studies in this category. The results without this study are shown in the final row of the table. The results remain similar to those including the study, although I2 increased slightly. July 2015 Page 45 Table 15. Summary of sensitivity analyses using the random effects model. Mean Correlation N of Heterogeneity Heterogeneity Heterogeneity coefficient studies 2 I2 (%) P-value CHX chip -0.1 6 0.00 0 0.54 0.41 CHX chip 0.9 6 0.04 81 <0.0001 0.32 CHX chip without Gonzales 0.25 5 0.01 28 0.24 0.39 Treatment 95% difference CI (mm) 0.25, 0.58 0.15, 0.50 0.21, 0.58 Evidence profile The summary of findings is shown in Table 16. The panel judged the overall level of certainty in the evidence to be moderate based on the quantity of evidence, the overall risk of bias, the consistency, and applicability of the data to U.S. populations. Table 16. Evidence profile for SRP+CHX chip versus SRP alone Level of certainty assessment Mean difference in CAL Precision (mm) Too few 0.40 higher SRP+CHX No serious 6 316 Unclear Consistent Yes studies to Moderate (0.24 to chip vs. SRP imprecision assess 0.56)* *Interpreted as 0.40 mm mean gain in clinical attachment level with SRP plus chlorhexidine chip use versus SRP alone, with a 95% Therapy Quantity of evidence No. No. RCTs participants Risk of bias Consistency Applicability Publication bias Level of Certainty CI from 0.24 mm to 0.56 mm improvement. Adverse events assessment (potential for harms) Two of the six included studies assessed and reported about adverse events. Dimitra et al.174 reported there were no patient-reported adverse effects, such as discomfort, pain or swelling, resulting from chlorhexidine chip placement. Heasman et al.171 reported one non-treatmentrelated aphthae on the buccal mucosa. According to FDA prescribing information, the most frequently observed adverse events were toothache, upper respiratory tract infection, and headache.182 The only adverse event with frequency of occurrence that was statistically significantly higher than the placebo group was toothache. Oral pain or sensitivity may occur during the first week after SRP and chip placement, although in some cases it may occur later, but it is typically mild to moderate in severity and is expected to resolve within days. Postmarketing surveillance reports that anaphylaxis, as well as serious allergic reactions, have occurred with dental products containing chlorhexidine.182 July 2015 Page 46 The panel notes that the products should be applied according to the manufacturers’ general instructions. Balancing benefits with potential for harms The panel judged that there is a balance between a moderate benefit and potential harms. 3.2.4.2 Doxycycline Hyclate Gel + SRP General description of studies Three small studies met inclusion criteria reporting the effect of SRP plus the local delivery of doxycycline hyclate gel compared to SRP alone.183-185 Two studies184, 186 reported on the same participants and are included as one set of data with preference given to the 6-month study184 results because more participants were included. Two124, 126 were split-mouth183, 185 and one184 was a parallel-group trial. The sample sizes ranged from 10 to 22 participants per group. One study was conducted in Turkey183 and the other two in Brazil.184, 185 The studies were conducted between 2004 and 2006. All participants in the Martorelli de Lima et al. study185 had Type I diabetes mellitus. The participants in two183, 185 of the studies were diagnosed with advanced chronic periodontitis, whereas the disease severity in the participants in the other study184 was not reported. Machion et al.184 included only smokers, whereas Agan et al.183 and Martorelli de Lima et al.185 did not describe the smoking status of the participants. Because of the variety of smoking inclusion/exclusion criteria, no further sub-analyses determined if the results were different for smokers versus non-smokers. One study was excluded because the concentration of doxycycline hyclate (14%) in the product is not commercially available in the U.S.187 One study188 reported on the effects of doxycycline hyclate gel in smokers, but the study was excluded because 1) it combined subpopulations from two randomized studies and an open label study with no control arm; and 2) the patients were included in other unpublished trials189 included in the analysis. Critical appraisal Details for each domain and the support for each judgment for each individual study are presented in Appendix 3. Only one study183 reported an adequate method of randomization, while the others did not describe the specific randomization method used. No studies reported methods for allocation concealment. In only one study185 were both the participants and clinicians masked; in one study183 it was unclear if both were masked; and in one study184 the July 2015 Page 47 clinicians were masked but not the participants. In all three studies the groups were treated the same except for the intervention. In one study184 the outcomes assessor was masked, whereas in the other two studies their masking was unclear. All studies had low risk of attrition bias. There was low risk of selective reporting bias. The judgments of high, unclear, or low risk for bias as a percentage of included studies in this section are depicted by domain in Figure 13. Because all domains of the studies are at low or unclear risk of bias, and the impact of those domains judged to be at unclear risk of bias on the summary estimate of effect is not known, the overall assessment for the risk of bias for this body of evidence was judged to be unclear. Figure 13. Review authors’ judgment of each risk of bias item as low, unclear, or high as a percent of included studies (there were 3 studies). Results of intervention Figure 14 shows the meta-analysis for the included studies comparing SRP to SRP plus doxycycline hyclate gel sub-grouped by study design. The overall group mean difference is 0.64 mm (95% CI, 0.00 to 1.28) mean gain in clinical attachment level (i.e., improvement) when doxycycline hyclate gel was used adjunctively with SRP versus SRP alone. Since there are only three trials with wide and overlapping confidence intervals, the effect of study design was not statistically significant (p=0.80). July 2015 Page 48 Figure 14. Meta-analysis of studies on SRP plus doxycycline hyclate gel versus SRP alone, subgrouped by study design; mean difference in units of millimeters. Notes: Martorelli de Lima, used endpoint data, and SE converted to SD for data adjustments; Agan, CAL gain reported but not SD, SE imputed by averaging SEs from the other two studies; Machion, used Machion (2004) 6-month data over Machion (2006) 12month data because n is higher for the earlier study, also used all pocket data over pocket-depth-stratified data; reported the mean difference and SD between baseline and final CAL so no r adjustment necessary. Sensitivity analyses Because assumptions were made with respect to choosing a correlation coefficient for the statistical calculations, the sensitivity of the result to these assumptions was investigated. Table 17 shows the impact of changing the correlation coefficient from the initial value of 0.25 to both 0.1 and 0.9. At a correlation coefficient of -0.1, the mean difference decreased slightly from 0.64 mm to 0.60 mm and no longer touched the line of no effect and statistical heterogeneity decreased. At a correlation of 0.9, the mean difference increased to 0.76 mm, but became more imprecise, crossing the line of no effect. Statistical heterogeneity also increased. One study (insert Agan) did not report an SD, so it was imputed. The results without this study are shown in the final row of the table. In this case, the mean difference increased to 0.93 mm and barely crossed the line of no effect. The mean difference is judged to be somewhat sensitive to the assumptions that were made. July 2015 Page 49 Table 17. Summary of sensitivity analyses using the random effects model. Treatment Hetero- Mean geneity P- difference (%) value (mm) 0.08 26 0.26 0.60 0.03, 1.17 3 0.44 86 0.001 0.76 -0.05, 1.57 2 0.28 55 0.14 0.93 -0.01, 1.86 Correlation N of Hetero- coefficient studies geneity 2 -0.1 3 0.9 0.25 Doxycycline hyclate gel Doxycycline hyclate gel Doxycycline hyclate gel without Agan et al. Heterogeneity I2 95% CI Evidence profile Table 18 summarizes the evidence profile for SRP + doxycycline hyclate gel versus SRP alone. The panel judged the overall level of certainty in the evidence to be low based on the quantity of evidence, the overall risk of bias, the consistency, and applicability of the data to U.S. populations. Table 18. Evidence profile for SRP+doxycycline hyclate gel versus SRP alone. Level of certainty assessment Therapy Quantity of evidence No. No. RCTs participants Risk of bias Consistency Applicability Precision Publication bias Level of Certainty SRP+ Serious Too few doxycycline Moderate 3 64 Unclear Yes imprecisio studies to Low hyclate gel vs. inconsistency n assess SRP *Interpreted as 0.64 mm mean gain in clinical attachment level with SRP plus doxycycline hyclate gel versus SRP alone, with a 95% CI from 0.00 mm to 1.28 mm improvement. Adverse events assessment (potential for harms) Adverse events were recorded from 1) included articles; 2) articles where the product was used for at least six months, but not as an adjunct to SRP; 3) articles without CAL data but with adverse event information; and 4) adverse events available from the FDA. From the included studies, adverse events either were not described by the authors or none were reported by the participants. Two excluded studies reported adverse reactions as follows: Wennstrom et al.190 reported that 34% of participants reported adverse events after the initial phase of treatment, but that there was no difference in the frequency of adverse events between the experimental and control arms of the trial. The majority of the reported events was related to July 2015 Page 50 Mean difference in CAL (mm) 0.64 higher (0.00 to 1.28) mild or moderate gingival soreness and thermal tooth sensitivity following treatment. Garrett et al.191 reported that a large majority of adverse events that were probably or possibly related to treatment were related to moderate gingival soreness following treatment, and that no subjects withdrew from the trial who were in the doxycycline hyclate gel arm. The authors noted that no subjects experienced oral candidiasis. The FDA label192 lists the following warnings for Atridox® (this is a partial list): May cause permanent discoloration of the teeth during tooth development (pregnant women, infancy, or childhood to age eight); Enamel hypoplasia reported; Photosensitivity manifested by sunburn reaction has been observed; avoid exposure to direct sunlight or ultraviolet light; May result in overgrowth of nonsusceptible microorganisms including fungi; Caution with a history of predisposition to oral candidiasis; other potential adverse reactions: headache, gum discomfort (pain/soreness), toothache, periodontal (abscess, exudate, infection, drainage, extreme mobility, suppuration), thermal tooth sensitivity, sore mouth, premenstrual tension syndrome, muscle aches, common cold, respiratory flu, stuffy head, post nasal drip, congestion, sore throat. The FDA label192 lists these adverse reactions from clinical trials of over 1,400 participants: 1.6% of participants on Atridox® reported “unspecified essential hypertension”, while only 0.2% in the vehicle control arm and none in either the SRP or oral hygiene instruction arms reported this adverse event. There is no known association of oral doxycycline hyclate use with essential hypertension. Two participants in the placebo group and none in the Atridox® group reported localized allergic response. This product has not been clinically tested in pregnant women or immunocompromised participants.192 Balancing benefits with potential for harms Since the benefits are unclear, the panel judged that there is uncertainty in the balance between benefits and harms. 3.2.4.3 Minocycline Microspheres + SRP General description of studies Three small87, 193, 194 and two relatively large and unpublished new drug application (NDA) studies (“Study 103A” and “Study 103B” available in one document189) met inclusion criteria reporting the effect of SRP plus the local delivery of minocycline microspheres compared to SRP alone. The sample sizes in the small studies ranged from 10-15 participants per group, July 2015 Page 51 whereas the unpublished study sample sizes ranged from 121-128 per group. One study was a split-mouth design,193 whereas the others were parallel-group. One study was conducted in New Zealand193, one in Slovenia194, and the others in the U.S. in the time period between 2000 and 2004. The severity of periodontitis was moderate to severe (or “advanced”) for all studies except one194, for which disease severity was not reported. Four studies included smokers but did not perform sub-analysis189, 193, 194; and one87 did not indicate the smoking status of the participants. All participants in Skaleric et al.194 had Type I diabetes. Critical appraisal Details for each domain and the support for each judgment for each individual study are presented in Appendix 3. None of the studies described the specific randomization method used or methods for allocation concealment. None of the studies masked the personnel, and only one clearly masked the participants. In all studies the groups were treated the same except for the intervention. In four of the five studies the outcomes assessor was masked, whereas in the other studies it was unclear. All studies had low risk of attrition bias due to less than 10% lost to follow-up, except one,87 in which case the risk to bias was judged to be unclear because of up to 20% lost to follow-up. There was uncertainty in the risk of selective reporting bias in two unpublished studies189 because CAL data were not included in the study publication195; however, CAL was not listed as the studies' primary outcome measure. Note that the critical appraisals for the two unpublished data sets189 are based on Williams et al.,195 since it contains methodological details that are not available in the unpublished source. The judgments of high, unclear, or low risk for bias as a percentage of included studies in this section are depicted by domain in Figure 15. Because most of the studies are at low or unclear risk of bias, and the impact of the lack of masking of participants and personnel on the summary estimate of effect, the overall assessment for the risk of bias for this body of evidence was judged to be unclear. July 2015 Page 52 Figure 15. Review authors’ judgment of each risk of bias item as low, unclear, or high as a percent of included studies. Note: Williams served as proxy for evaluating the methodology of two unpublished NDA reports (four studies in this assessment). Results of intervention Figure 16 shows the meta-analysis for the included studies comparing SRP to SRP plus minocycline microspheres sub-grouped by study design. The overall group mean difference is 0.24 mm mean gain in clinical attachment level (i.e., improvement) when minocycline microspheres were used adjunctively with SRP versus SRP alone with a 95% CI from -0.06 to 0.55 mm. The effect of study design was not shown to be statistically significant. With respect to split-mouth trials, there can be concern about a carryover effect between treated and untreated sites in the same mouth, which would create a bias toward the null (a conservative bias). Henderson et al.193, however, compared the effect of minocycline on adjacent and remote sites, and concluded there was no carryover effect. July 2015 Page 53 Figure 16. Meta-analysis of studies on SRP plus minocycline microspheres versus SRP alone, sub-grouped by study design; mean difference in units of millimeters. Notes: Henderson, used n at baseline (final n not reported), and combined control groups (adjacent and remote sites); Studies 103A and 103B reported the mean difference and SD between baseline and final CAL so no adjustment necessary; Van Dyke, used all treated sites data although data stratified by pocket depth are available, and also converted SE to SD, reported the mean difference and SD between baseline and final CAL so no adjustment necessary; Skaleric, change score and time correlation calculated. Sensitivity analyses Because assumptions were made with respect to choosing a correlation coefficient for the statistical calculations, the sensitivity of the result to these assumptions was investigated. Table 19 shows the impact of changing the correlation coefficient from the initial value of 0.25 to both 0.1 and 0.9. At a correlation of -0.1, the mean difference decreased from 0.24 mm to 0.16 mm and still crossed the line of no effect. At a correlation coefficient of 0.9, the mean difference increased to 0.49 mm, and no longer crossed the line of no effect. The mean difference is judged to be sensitive to the assumptions that were made, contributing to the low level of certainty in the evidence. Table 19. Summary of sensitivity analyses using the random effects model. Treatment Minocycline microspheres Minocycline microspheres July 2015 Correlation coefficient N of studies Heterogeneity 2 Heterogeneity I2 (%) Heterogeneity P-value Mean difference (mm) -0.1 5 0.01 14 0.32 0.16 0.9 5 0.20 83 <0.0001 0.49 95% CI -0.08, 0.41 0.05, 0.93 Page 54 Evidence profile Table 20 summarizes the panel's findings. The panel judged the overall level of certainty in the evidence to be low based on the quantity of evidence, the overall risk of bias, the consistency, and applicability of the data to U.S. populations. Table 20. Evidence profile for SRP+minocycline microspheres versus SRP alone. Level of certainty assessment Therapy Quantity of evidence No. No. RCTs participants Risk of bias Consistency Applicability Precision Level of Certainty Publication bias SRP + minoToo few cycline Moderate Serious 5 572 Unclear Yes studies to Low microspheres inconsistency imprecision assess vs. SRP *Interpreted as 0.24 mm mean gain in clinical attachment level with SRP plus minocycline microspheres versus SRP alone, with a 95% CI from 0.06 mm worsening to 0.55 mm improvement. Adverse events assessment (potential for harms) Adverse events were recorded from 1) included articles; 2) articles where the product was used for at least six months, but not as an adjunct to SRP; 3) articles without CAL data, but with adverse event information; and 4) adverse events available from the FDA. From the included studies, Van Dyke et al.87 reported one adverse event (black hairy tongue) that was ruled to be possibly drug-related. Other adverse events were reported but judged not to be related to study medication. There was no significant difference in intensity and extent of tooth staining at any assessment point between groups that received or did not receive minocycline. There were no abnormal clinical chemistry or haematological results observed in the study for any of the groups. Williams et al.195 (the CAL data from which are included in Studies 103A189 and 103B189) reported no dramatic differences between groups. Adverse events were reported by 62.4% and 68.3% of participants in control and combination therapy groups respectively. The incidence of adverse events was similar among treatment groups. The most common adverse events included headache, dental infection, increased periodontitis, tooth sensitivity, tooth caries, dental pain, gingivitis, and stomatitis. No clinically significant changes in vital signs or oral hard or soft tissues were noted in these studies. The FDA label196 lists the following warnings for Arestin® (this is a partial list, and includes warnings for the tetracycline class of drugs): May cause permanent discoloration of the teeth July 2015 Page 55 Mean difference in CAL (mm) 0.24 higher (-0.06 to 0.55)* during tooth development; enamel hypoplasia reported; special consideration should be given to pregnant women; photosensitivity manifested by sunburn reaction has been observed, avoid exposure to direct sunlight or ultraviolet light. Special precautions include hypersensitivity reactions not limited to anaphylaxis and serious skin reactions, such as Stevens Johnson syndrome (SJS) and erythema multiforme reported; associated with development of autoimmune syndromes including a lupus-like syndrome. Not recommended in an acutely abscessed periodontal pocket. May result in overgrowth of nonsusceptible microorganisms including fungi; caution is advised for those with a history of predisposition to oral candidiasis. The FDA label196 lists the following most frequently reported adverse events in three multicenter U.S. trials: headache, infection, flu syndrome, and pain. The panel notes that the products should be applied according to the manufacturers’ instructions. Balancing benefits with potential for harms Since the benefits are unclear, the panel judged that there is uncertainty in the balance between benefits and harms. 3.2.5 Nonsurgical use of lasers + SRP The panel of experts analyzed all studies that met the inclusion criteria of nonsurgical application of a laser (pocket disinfection) and did not consider studies using lasers for alternative surgical therapy. There are several lasers used nonsurgically as adjunctive treatments with SRP. The lasers are categorized primarily by the wavelength of the emitted light. Five categories of lasers are included and described below. One laser type was not available in the U.S. (potassium titanyl phosphate, KTP) 197 and not included. The panel notes that there are no standard operating protocols (such as power intensity/density, power, spot size, energy, repetition rate, tip size, pulsing vs. continuous mode, mean energy loss, or time of application) for the lasers. 3.2.5.1 PDT diode laser + SRP General description of studies Ten studies198-207 met inclusion criteria reporting the effect of SRP plus a photodynamic-therapy (PDT) diode laser (wavelength 660-810 nm) versus SRP alone. Six198, 201-204, 206 were split-mouth trials, and four199, 200, 205, 207 were parallel-group. The sample sizes were relatively small, ranging from 12 to 44 per treatment group. The studies were conducted worldwide including three in July 2015 Page 56 Brazil, two in the Netherlands, and one each in Germany, India, Italy, Jordan, and Turkey. The studies were published between 2008 and 2014. Two of the studies201, 202 described the severity of disease of included participants as moderateto-severe, seven197-200, 204, 205, 207 did not describe the severity except by probing pocket depth and bleeding on probing inclusion criteria, and one study203 reported on slight-to-moderate periodontitis in participants with HIV. Five studies excluded smokers,197, 198, 202, 205, 207 four199-201, 203 included smokers, but did not perform a sub-group analysis reporting the results by smoking status, and one did not mention smoking status.204 It is noted that Theodoro only included one site per treatment. Two arms of two studies were excluded: the toluidine blue O (TBO)-only arm of Theodoro et al.202 and the KTP laser arm of Dilsiz et al.197 Critical appraisal Details for each domain and the support for each judgment for each individual study are presented in Appendix 3. Nine of ten studies were at a low risk of bias for random sequence generation, and identical treatment of groups except for the intervention; and all ten studies were at a low risk of bias for masking of outcomes assessment and detection of selective reporting. The judgments of high, unclear, or low risk for bias as a percentage of included studies in this section are depicted by domain in Figure 17. The main issues for bias for this group of studies were lack of masking of personnel and participants, which may have been difficult to obtain due to the nature of the intervention. The risk of bias for these two domains were judged as not being as significant as outcomes assessment masking. The panel judged the overall risk of bias of the included studies as low. July 2015 Page 57 Figure 17. Review authors’ judgment of each risk of bias item as low, unclear, or high as a percent of included studies. Assessment of publication bias Since there were 10 studies included comparing PDT lasers plus SRP to SRP alone, an assessment of publication bias by visual inspection of the funnel plot as well as Egger’s test were undertaken. Figure 18 shows the funnel plot with the studies identified by their sub-group. The panel found no evidence for publication bias (Egger’s test, p=0.679). Figure 18. Funnel plot of studies on SRP plus PDT lasers. .2 .3 .5 .4 SE Mean Difference .1 0 Funnel plot with pseudo 95% confidence limits -1 0 1 2 Mean Difference July 2015 Page 58 Results of intervention Figure 19 shows the meta-analysis for the included studies comparing SRP to SRP plus PDT diode laser. The overall group mean difference is 0.53 mm mean gain in clinical attachment level (i.e., improvement) when a PDT diode laser was used adjunctively with SRP versus SRP alone with a 95% CI from 0.06 mm to 1.00 mm improvement. The subgroup analysis indicated that the results were not statistically significantly different with respect to study design. Figure 19 (updated). Meta-analysis of studies on SRP plus PDT diode laser versus SRP alone, subgrouped by study design; mean difference in units of millimeters. Notes: Gianelli, final data used; Berakdar, mean CAL gains visually estimated from publication figure and SD estimated by dividing range by 2; Filho, final data used (change score not provided); Theodoro, final data used (change score not provided); Dilsiz, change data used; Betsy, SE estimated by averaging since only intraquartile range reported; Alwaeli, Christodoulides, Chondros, and Luchesi, no adjustments necessary. Sensitivity analyses Because assumptions were made with respect to choosing a correlation coefficient for the statistical calculations, the sensitivity of the result to these assumptions was investigated. Table 21 shows the impact of changing the correlation coefficient from the initial value of 0.25 to both 0.1 and 0.9. At both correlation coefficients -0.1 and 0.9, the mean difference and 95% CI did not change appreciably from those found at the initial value of 0.25. At all correlation coefficients, there is true between-study variation. The summary estimate is judged to not be sensitive to the assumptions that were made. July 2015 Page 59 Table 21. Summary of sensitivity analyses using the random effects model. Hetero- Mean geneity p- difference (%) value (mm) 0.49 84 <0.00001 0.52 0.03, 1.02 10 0.46 95 <0.00001 0.53 0.09, 0.97 9 0.51 87 <0.00001 0.47 -0.04, 0.99 Adjunctive Correlation # of Hetero- Treatment coefficient studies geneity 2 -0.1 10 0.9 0.25 PDT diode laser PDT diode laser Without Betsy (estimated SE) Heterogeneity I2 95% CI Evidence profile The evidence profile is summarized in Table 22. The panel judged the overall level of certainty in the evidence to be low based on inconsistency and imprecision. Table 22. Evidence profile for SRP+PDT diode laser versus SRP alone. Level of certainty assessment Therapy Quantity of evidence No. No. RCTs participants Risk of bias Consistency Applicability Precision Publication bias Level of Certainty SRP+ PDT None Serious diode laser 10 306 Low Inconsistent Yes detected Moderate imprecision vs. SRP (p=0.679) *Interpreted as 0.48 mm mean gain in clinical attachment level with PDT diode laser adjunctive use with SRP versus SRP alone, with a 95% CI from 0.05 mm to 0.92 mm improvement. Adverse events assessment (potential for harms) Several studies reported no adverse events: therapies were well tolerated198, healing was uneventful with no pain or discomfort reported199, no burning sensation or pain with laser treatment200, 205, no major periodontal inflammatory symptoms after instrumentation during the entire study or complications such as infections, suppuration, or abscesses were observed197, 204 , and no complications201-203 or adverse effects207. Balancing benefits with potential for harms Uncertainty in magnitude of moderate benefit balanced with potential harms. July 2015 Page 60 Mean difference in CAL (mm) 0.53 higher (0.06 to 1.00)* 3.2.5.2 Non-PDT diode laser + SRP General description of studies Four studies208-211 met inclusion criteria reporting the effect of SRP plus a non-photodynamictherapy (non-PDT) diode laser (wavelength 808 to 980 nm). Three were split-mouth, and one209 was a parallel group study. In Euzebio Alves et al.208 only one site per mouth was tested with each treatment. The sample sizes were relatively small, between 13 and 36. The studies were conducted in Brazil,208 Italy,211 and Turkey,209, 210 and published in 2008 through 2014. One208 of the studies described the severity of disease of the included participants (severe chronic periodontitis), while the others were judged to include moderate severity based on the probing pocket depth inclusion criteria. Three of the studies excluded smokers while Caruso et al.211 did not describe the smoking status of their participants. Because of the variety of smoking inclusion/exclusion criteria, no further sub-analyses to determine if the results were different for smokers versus non-smokers were attempted. Critical appraisal Details for each domain and the support for each judgment for each individual study are presented in Appendix 3. Euzebio Alves et al.208 was at a low risk of bias for all dimensions. The judgments of high, unclear, or low risk for bias as a percentage of included studies in this section are depicted by domain in Figure 20. Because both allocation concealment and participant, personnel, and outcomes assessment masking were unclear for one211 of the studies, the fact that only one site was treated in each arm of Alves et al.208, and the lack of participant and personnel masking in two studies209, 210, the panel judged the risk of bias for the two studies as a whole as unclear. July 2015 Page 61 Figure 20. Review authors’ judgment of each risk of bias item as low, unclear, or high as a percent of included studies. Results of intervention Figure 21 shows the meta-analysis for the included studies comparing SRP to SRP plus nonPDT diode laser. The overall group mean difference is 0.21 mm mean gain in clinical attachment level when a non-PDT laser was used adjunctively with SRP treatment versus SRP alone with a 95% CI from 0.23 mm worse to 0.64 mm improvement. Because the 95% CI crosses the line of no effect, this result is interpreted as no statistically significant difference between the two treatments. Figure 21. Meta-analysis of studies on SRP plus non-PDT laser versus SRP alone; mean difference in units of millimeters. July 2015 Page 62 Notes: Caruso, final values were used (not change over time); Alves, CAL gain was reported as well as SD; Ustun, final values used; Saglam, change score calculated. Sensitivity analyses Because assumptions were made with respect to choosing a correlation coefficient for the statistical calculations, the sensitivity of the result to these assumptions was investigated. Table 23 shows the impact of changing the correlation coefficient from the initial value of 0.25 to both 0.1 and 0.9. At a correlation of -0.1 and 0.9, the mean difference still indicates no statistically significant effect from adding the adjunctive treatment. Table 23. Summary of sensitivity analyses using the random effects model. Treatment Correlation coefficient # of studies Heterogeneity 2 Heterogeneity I2 (%) Heterogeneity p-value Mean difference (mm) -0.1 4 0.06 33 0.21 0.21 0.9 4 0.19 93 <0.00001 Non-PDT diode laser Non-PDT diode laser 95% CI -0.22, 0.64 -0.25, 0.63 0.19 Evidence profile The evidence profile is summarized in Table 24. The panel judged the overall level of certainty in the evidence to be low based on the small quantity of evidence, the unclear risk of bias, the substantial inconsistency, and serious imprecision. Table 24. Evidence profile for SRP+non-PDT laser versus SRP alone. Level of certainty assessment Therapy Quantity of evidence No. No. RCTs participants Risk of bias Consistency Applicability Precision Publication bias Level of Certainty SRP+nonSubstantial Too few Serious PDT diode 4 98 Unclear inconsisten Yes studies to Low imprecision laser vs. SRP cy assess *Interpreted as 0.21 mm mean gain in clinical attachment level with non-PDT diode laser adjunctive use with SRP versus SRP Mean difference in CAL (mm) 0.21 (-0.23 to 0.64)* alone, with a 95% CI from 0.23 mm worse to 0.64 mm improvement. Adverse events assessment (potential for harms) Two studies reported no adverse events such as discomfort, burning sensation, dentin hypersensitivity, or pain related to non-PDT laser irradiation. . Balancing benefits with potential for harms There was no evidence of a benefit. July 2015 Page 63 3.2.5.3 Nd:YAG laser + SRP General description of studies Three studies81, 212, 213 met inclusion criteria reporting the effect of SRP plus an Nd:YAG laser (wavelength 1064 nm). All were split-mouth studies with small sample sizes (10 to 26 participants). One was conducted in the U.S. in 1997 on participants with moderate-to-severe periodontitis81, while another was conducted in Turkey in 2012 on participants with moderate periodontitis212. Neither of these studies mentioned the smoking status of the included participants. One study213 compared the effects of the addition of Nd:YAG lasers to SRP on smokers versus nonsmokers in two arms of the study. Critical appraisal Details for each domain and the support for each judgment for each individual study are presented in Appendix 3. The judgments of high, unclear, or low risk for bias as a percentage of included studies in this section are depicted by domain in Figure 22. The main issues for bias for this group of studies were lack of or unclear masking of personnel, participants, and outcomes assessors. The panel judged the overall risk of bias of the included studies as unclear. Figure 22. Review authors’ judgment of each risk of bias item as low, unclear, or high as a percent of included studies. Results of intervention Figure 23 shows the meta-analysis for the included studies comparing SRP to SRP plus Nd:YAG laser. The overall group mean difference is 0.41 mm mean gain in clinical attachment July 2015 Page 64 level when an Nd:YAG laser was used adjunctively with SRP treatment versus SRP alone with a 95% CI from 0.12 mm worsening to 0.94 mm improvement. Figure 23. Meta-analysis of studies on SRP plus Nd:YAG laser versus SRP alone; mean difference in units of millimeters. Notes: Neill, used data from text, which appears to be inconsistent from publication Figure 4; Eltas A and B, final data used (change score not provided). Sensitivity analyses Because assumptions were made with respect to choosing a correlation coefficient for the statistical calculations, the sensitivity of the result to these assumptions was investigated. Table 25 shows the impact of changing the correlation coefficient from the initial value of 0.25 to both 0.1 and 0.9. At a correlation coefficient of -0.1 the mean difference and 95% CI did not change from the original value, and at a correlation coefficient of 0.9, the mean difference and 95% CI decreased slightly. Since there were only three studies, one of which had two arms, heterogeneity statistics are less meaningful. However, the trend is for heterogeneity to increase with increasing correlation coefficient values. The summary estimate is judged to not be sensitive to the assumptions that were made. Table 25. Summary of sensitivity analyses using the random effects model. Adjunctive Treatment Correlation coefficient # of studies Heterogeneity 2 Heterogeneity I2 (%) Heterogeneity p-value Mean difference (mm) Nd:YAG laser -0.1 3 0.13 46 0.13 0.41 Nd:YAG laser 0.9 3 0.27 95 <0.00001 0.40 July 2015 Page 65 95% CI -0.12, 0.94 -0.12, 0.93 Evidence profile The evidence profile is summarized in Table 26. The panel judged the overall level of certainty in the evidence to be low based on the small quantity of evidence, the unclear risk of bias, and inconsistent results. Table 26. Evidence profile for SRP+Nd:YAG laser versus SRP alone. Level of certainty assessment Therapy Quantity of evidence No. No. Studies participants Risk of bias Consistency Applicability Precision Publication bias Level of Certainty Too few studies to Moderate Serious assess; expert 3 82 Unclear Yes Low inconsistency imprecision knowledge of unpublished studies *Interpreted as 0.41 mm mean gain in clinical attachment level with Nd:YAG laser adjunctive use with SRP versus SRP alone, with SRP+ Nd:YAG laser vs. SRP a 95% CI from 0.12 mm worsening to 0.94 mm improvement. Adverse events assessment (potential for harms) One study stated that there were no adverse events reported as well as minimal pain81, the other two studies212, 213 did not include adverse event reporting. Balancing benefits with potential for harms There was no evidence of a benefit. 3.2.5.4 Erbium laser + SRP General description of studies Three studies83, 214, 215 met inclusion criteria reporting the effect of SRP plus an erbium laser (either Er,Cr:YSGG214 or Er:YAG83, 215, wavelengths 2.79 and 2.94 m, respectively). All were split-mouth studies with small sample sizes (19-33 participants). Each study was conducted in a different country: Lithuania214, Brazil215, and Italy83, and published between 2010 and 2011. Two of the studies83, 215 described the severity of disease of included participants as moderateto-severe, and one214 was early-to-moderate. Two of the studies214, 215 excluded smokers, and one83 included smokers but did not perform a sub-group analysis reporting the results by smoking status. July 2015 Page 66 Mean difference in CAL (mm) 0.41 higher (-0.12 to 0.94)* Critical appraisal Details for each domain and the support for each judgment for each individual study are presented in Appendix 3. All studies were at low risk of bias for random sequence generation. One83 was at low risk of bias for allocation concealment, while the others did not report details about their methods in this area. Masking of participants and personnel was either unclear or not performed; however, in all studies the outcomes assessor was masked. There was low risk of bias for all studies with respect to identical treatment of groups except for the intervention, selective reporting, and complete outcomes data, except for one study214 where there was an unclear risk of bias concerning incomplete outcomes data. The judgments of high, unclear, or low risk for bias as a percentage of included studies in this section are depicted by domain in Figure 24. The main issues for bias for this group of studies were lack of masking of personnel and participants, which may have been difficult to obtain due to the nature of the intervention. This risk of bias was judged as not being as significant as outcomes assessment masking. The panel judged the overall risk of bias of the included studies as low. Figure 24. Review authors’ judgment of each risk of bias item as low, unclear, or high as a percent of included studies. Results of intervention Figure 25 shows the meta-analysis for the included studies comparing SRP to SRP plus an erbium laser. The overall group mean difference is 0.18 mm mean gain in clinical attachment level (i.e., improvement) when an erbium laser was used adjunctively with SRP versus SRP alone with a 95% CI from 0.63 mm worsening to 0.98 mm improvement. These results are July 2015 Page 67 interpreted as a non-statistically-significant difference from SRP with the addition of an erbium laser. Figure 25. Meta-analysis of studies on SRP plus erbium laser versus SRP alone; mean difference in units of millimeters. Notes: Rotundo, final data used (change score not provided); Lopes, final data used (change score not provided); Kelbauskiene, change scores provided and used. Sensitivity analyses Because assumptions were made with respect to choosing a correlation coefficient for the statistical calculations, the sensitivity of the result to these assumptions was investigated. Table 27 shows the impact of changing the correlation coefficient from the initial value of 0.25 to both 0.1 and 0.9. At both correlation coefficients -0.1 and 0.9, the mean difference and 95% CI did not change appreciably from those found at the initial value of 0.25. There is less statistical heterogeneity at the smaller correlation coefficient. The summary estimate is judged to not be sensitive to the assumptions that were made. Table 27. Summary of sensitivity analyses using the random effects model. Adjunctive Treatment Correlation coefficient # of studies Heterogeneity 2 Erbium laser Erbium laser -0.1 0.9 3 3 0.32 0.47 Heterogeneity I2 (%) 64 96 Heterogeneity pvalue 0.06 <0.00001 Mean difference (mm) 0.20 0.13 95% CI -0.61, 1.01 -0.66, 0.92 Evidence profile The evidence profile is summarized in Table 28. The panel judged the overall level of certainty in the evidence to be low based on the small quantity of evidence, inconsistent results, and evidence of no effect. July 2015 Page 68 Table 28. Evidence profile for SRP+erbium laser versus SRP alone. Therapy Quantity of evidence No. No. RCTs participants Level of certainty assessment Risk ConsistAppliof Precision ency cability bias Publication bias Level of Certainty Mean difference in CAL (mm) Too few 0.18 higher studies to Low (-0.63 to assess 0.98)* *Interpreted as 0.18 mm mean gain in clinical attachment level with SRP plus erbium laser adjunctive use versus SRP alone, with a SRP+ erbium laser vs. SRP 3 82 Low Inconsistent Yes Serious imprecision 95% CI from 0.63 mm worsening to 0.98 mm improvement. Adverse events assessment (potential for harms) One study83 reported the occurrence of abscesses, three in the control group and two in the laser group. There was one patient who reported fever in the week following treatment, but the treatment group was not identified. 83 The other two studies214, 215 did not report on adverse events. Balancing benefits with potential for harms There was no evidence of a benefit. 3.2.5.5 Summary statements on non-surgical use of lasers Lasers, unlike other instrumentation, have no defined and accepted protocols for standard usage. Since every operator determines their own protocol based on anecdotal rules or experiences, the potential for harms to the tooth and patient is higher than other local delivery systems. Also, every laser wavelength is different and affects the hard and soft tissues differently, making comparisons between lasers unpredictable and often incorrect. Common protocols are needed for each laser used in nonsurgical therapy of chronic periodontitis to allow for repeatable results and comparisons among studies in the literature. The wide ranges found in the few studies considered for CAL gain/loss demonstrates the need for larger sample sizes and additional studies to properly evaluate the potential benefits of laser use as an adjunct to SRP. At this time, based on the criteria set in this systematic review there is insufficient evidence with any laser wavelength except PDT diode lasers to accurately define the benefits for adjunctive nonsurgical therapy of periodontitis with evidence-based literature. 3.3 Caries data Although the panel was interested in the effect that nonsurgical treatments for chronic periodontal disease have on caries, no data were found on this topic to answer the question. July 2015 Page 69 3.4 Smokers versus non-smokers Although some studies included only smokers or non-smokers or did post-hoc analyses comparing smokers to non-smokers, the panel was unable to make any general conclusions regarding the effect of any of the treatments on smokers versus non-smokers. 4. Discussion SRP is considered the gold standard for nonsurgical periodontal therapy. Because of SRP’s status, many clinical trials use SRP as an “active” control. This approach often leads to a clinical trial that compares the results from SRP to the results of a studied experimental therapeutic approach. Because there are very few trials that compare SRP to no treatment, the panel was limited in their ability to determine the level of certainty above the level of “moderate”. The panel found multiple trials for sub-antimicrobial dose doxycycline. By definition, subantimicrobial dose doxycycline is prescribed at a dosage that does not show antimicrobial effect and is therefore not considered a systemic antibiotic. Sub-antimicrobial dose doxycycline showed a small and statistically significant adjunctive benefit. Since the dose is low, the panel judged that the small benefit outweighs the potential for harms. The panel found a variety of systemic antimicrobials and regimens used in periodontal treatment trials. Overall, the panel found a statistically significant but small benefit based on the class of antibiotics with variation between the subclasses of antibiotics. Since there is wide reporting of adverse events associated with systemic antibiotic use, the panel judged that the small benefit is balanced with the potential for harms. The panel observed a statistically significant, moderate benefit with the adjunctive use of CHX chips; however, the panel judged that these benefits were balanced with the potential for harms. The panel observed a substantial adjunctive benefit for doxycycline hyclate gel (DH); however, because of wide confidence interval around the estimated benefit, the data were also compatible with no benefit. It is important to note that DH was developed, originally tested, and FDA approved as a stand-alone product (i.e., used without scaling and root planing). Garret et al.132, 216 does address the use of DH as an independent therapy without SRP. Although there may be benefit for this independent approach, it was not a consideration for this systematic review, which addresses the benefit of DH as an adjunct to SRP versus SRP alone. Garret et al.132, 216 reported results from two large multi-center trials in which DH alone resulted in a 0.3 to July 2015 Page 70 0.5 mm gain in CAL [each study reported different results] when compared to no treatment, which was statistically significantly better than no treatment. They also presented results comparing DH to SRP, which indicated these treatments were not statistically significantly different from each other. The panel also observed a small adjunctive benefit for minocycline microspheres. Again, based on the confidence interval, the data for the microspheres also were compatible with no benefit. Additionally, minocycline microspheres were approved by the FDA for their beneficial effect on pocket depth (PD), not CAL. The panel did not assess PD change in this meta-analysis, although this measure may be of importance for some practitioners and patients. Overall, the small number of studies on both DH and minocycline microspheres limited the assessment of the consistency of studies, the usefulness of the sensitivity analyses, and the ability to assess publication bias. In addition, the risk of bias for both adjuncts was judged to be unclear. These factors add to the uncertainty of the estimated benefit for DH and minocycline microspheres. Clinicians should bear in mind the ambiguity of the adjunctive benefits of these products, represented by the wide confidence intervals, before recommending their use as part of the nonsurgical treatment of periodontitis. Lastly, lasers, unlike other instrumentation, have no defined and accepted protocols for standard usage. Since every operator determines their own protocol based on anecdotal rules or experiences, the potential for harms to the tooth and patient is higher than for other adjunctive treatment systems. Also, every laser type and wavelength is different and affects the hard and soft tissues differently, making comparisons between lasers unpredictable and potentially incorrect. Common protocols are needed for each laser used in nonsurgical therapy of chronic periodontitis to allow for repeatable results and valid comparisons among studies in the literature. The wide ranges found in the few studies considered for CAL gain/loss demonstrates the need for larger sample sizes and additional studies to properly evaluate the potential benefits of laser use as an adjunct to SRP. At this time, based on the criteria set in this systematic review, there is low certainty in the evidence for any laser type or wavelength except PDT diode lasers to accurately define the benefits of adjunctive nonsurgical therapy of periodontitis with evidence-based literature. Since there was imprecision in the magnitude of the benefit of PDT diode lasers, the panel judged there was a balance between the benefit and harm. July 2015 Page 71 5. Limitations 5.1 Of the evidence The amount of published material on the nonsurgical treatment of periodontitis is voluminous. However, this systematic review was limited by the number of studies that could be included in the review. Much of this limitation was due to the ambiguity inherent in many of the studies. As mentioned before, there were multiple studies that did not clearly specify that root planing was performed. Various descriptions were used such as debridement that may have been used interchangeably with root planing but the panel felt that the non-specificity of these terms had to preclude the inclusion of these studies. The literature is also inconsistent on what is a clinically relevant outcome: the value of the clinically relevant difference in attachment gain varied between 0.2 and greater than 1.0 mm. Another limiting factor was the description of the periodontal disease state of the patients. Part of this ambiguity was due to the fact that in the last several decades the description of the degree of periodontal disease has undergone several revisions. The panel made several exceptions to the description of chronic periodontal disease. However, many studies used outmoded or unconventional descriptions of the disease level of the patients included in these studies. The panel felt that the use of terms such as aggressive periodontitis was sufficiently at variance to the term chronic periodontitis that studies using this description could not be included. Also, many studies did not include any description of the disease state of the patients treated. In the future, the panel strongly urges researchers to include a standardized description of the level of disease being treated. Many otherwise rigorous studies reported their results in terms of pocket depth and not attachment levels. While pocket depths are the routine clinical measure used in most day-today treatment of patients, pocket depths do not recognize the role of recession in the treatment of periodontal diseases. Impressive reductions in pocket depth can be obtained through treatment-induced recession. With the use of CAL the reader can get a true measure of exactly how much the improvement was due to gain in attachment to the root surface as opposed to a reduction in pocket depth due to recession. Since the panel chose to rely on CAL, studies that only provide results in terms of pocket depth were excluded from this systematic review. Most of the included studies were small in terms of the number of participants. Small studies can have a problem with low statistical power. Several of the included studies tested only one July 2015 Page 72 site per patient per treatment. This procedure does not allow an adequate assessment of variation within a patient. A major concern in judging the reliability of the results of a study is the extent of patients that start the study and are retained to the end of the study. If a large number of patients drop out of a study before the final data is collected, this attrition can greatly influence the reliability of the results. Many studies did not include data about the retention of test subjects and this ambiguity in turn influenced the panel’s ability to judge the strength of the study’s findings. Although we intended to study the effect of these treatments on caries risk, we were unable to do so because few if any studies assessed this outcome. Also, issues regarding safety and/or adverse events were not often reported. The methodology in the literature on adjunctive use of lasers has generally been poorly reported and has not been standardized. The energy densities (i.e. dose of energy) were not standardized so methodological heterogeneity is introduced that complicates analysis and interpretation of the data. Techniques of application were also underreported. 5.2 Of the systematic review For this systematic review, only English language databases were searched and only articles in English were included in the review. These choices could lead to bias in the results and interpretations if significant studies published in languages other than English exist since they were not captured. In addition, by screening the literature according to inclusion/exclusion criteria, by definition studies were excluded that could provide additional information related to the topic, although the impact of these a priori decisions on the results is unknown. For example, many studies were excluded for being less than 6 months in duration or for reporting PD in preference to CAL. Although the disease severity information was captured during the data abstraction process, this systematic review did not assess the results subgrouped by disease severity. The competitive environment in which clinical trials are financed and conducted, as well as the non-reporting of negative results by some investigators or publications, fosters publication bias.217 Ten or more studies were identified in only three treatments in this systematic review allowing investigation of publication bias, so for the other treatments, the presence of publication bias is unknown. July 2015 Page 73 6. Future research The panel noted the need for further well designed studies that evaluate all types of adjunctive therapy. Many studies had specific design flaws, and many were inadequately powered. Poor methodology left the panel in the position of evaluating a particular adjunctive treatment from a minimum number of papers. This shortcoming can only be overcome by further well designed and powered studies in all areas of adjunctive treatment. The panel makes the following specific recommendations regarding the methodology of future trials: Methodology and reporting of future clinical trials should conform to current standards for human clinical trials. In part this protocol involves registering trials with clinicaltrials.gov and conforming to the CONSORT statement for human trials.218 These protocols generally ensure a minimum power to the study as well as speaking to treatment allocation, concealment (blinding) of evaluators and patients, bias in detection of outcomes, as well as attrition bias and reporting bias. To minimize attrition bias, researchers should focus more heavily on retaining enrolled participants and providing adequate personnel and resources to ensure that the primary outcome is assessed. Conduct larger, multi-center studies Test more than one site in a patient’s mouth Follow up patients at least 6 months Clearly define and describe endpoints that are reproducible Report mean CAL and variance, preferable in tables and not figures, and include baseline and final measurements or changes; consider additional summary statistics such as distributions or percent of sites improving Use parallel group trial design if at all possible. Only in specific circumstances would split-mouth trials be recommended Use appropriate statistical tests including checking for normal distribution of data and transforming skewed data Report details regarding statistical analysis methods and reasoning Report on tooth loss as well as post-treatment morbidity and recession Report on patient-centered outcomes (e.g., discomfort, oral health-related quality of life, satisfaction) and costs Use standardized protocols for handling failing sites and report the number of failing or retreated sites July 2015 Page 74 Evaluate safety or adverse events of treatments For laser studies, completely report or otherwise calculate all parameters/settings used such as energy density, duration of exposure, surface area of exposure, wavelength, frequency, time, hard/soft tissue, pulse and all other relevant techniques of application The panel also identified many topics for future studies related to nonsurgical treatment of chronic periodontitis: Studies comparing treatment results in smokers versus non-smokers Studies reporting effect of periodontal treatment on caries Studying the effect of irrigants without retreating failing sites with the test treatment during the trial Studies on special populations such as diabetics Studies investigating mechanisms of action of lasers to determine the relative importance of de-epithialization, antibacterial activity, or biostimulation Studies on CO2 lasers not delivered through fiber optic and KTP lasers that are not currently available in the U.S. Systematically reviewing the evidence on stand-alone treatments such as doxycycline hyclate gel in comparison to mechanical therapies such as SRP and not strictly as adjuncts to SRP Studying the effect of treatments on patients with varying severity of periodontitis to allow for more individualized care Systematically reviewing the evidence on debridement (scaling without root planing) on chronic periodontitis 7. Conclusions A total of 72 RCTs have been critically appraised and summarized on 10 forms of nonsurgical treatment for chronic periodontitis. The literature on SRP versus no treatment or debridement is scant, but confirmed the commonly reported36, 51 result of approximately 0.5 mm improvement in CAL. The literature on adjunctive therapies was varied providing only a moderate level of certainty on the benefits of the four adjunctive therapies: systemic sub-antimicrobial dose doxycycline, systemic antimicrobials, chlorhexidine chips, and photodynamic therapy with a diode laser. There was a low level of certainty on the benefits of all other adjunctive therapies. The panel also assessed the balance between the benefits and potential for adverse events and July 2015 Page 75 harms from each treatment. The panel makes specific recommendations for additional research on the topic of nonsurgical treatment for chronic periodontal disease to fill the gaps in knowledge and improve the evidence base. The panel makes clinical recommendations in a companion clinical practice guideline. July 2015 Page 76 8. References 1. Thornton-Evans G, Eke P, Wei L, et al. Periodontitis among adults aged >/=30 years - United States, 2009-2010. MMWR Surveill Summ 2013;62 Suppl 3:129-35. Armitage G. Development of a Classification System for Periodontal Diseases and Conditions. Ann Periodontol 1999;4:1-6. Golub LM, Wolff M, Lee HM, et al. Further evidence that tetracyclines inhibit collagenase activity in human crevicular fluid and from other mammalian sources. J Periodontal Res 1985;20:12–23. Golub LM, Ciancio S, Ramamurthy NS, Leung M, McNamara TF. Low-dose doxycycline therapy: Effect on gingival and crevicular fluid collagenase activity in humans. J Periodontal Res 1990;25:321–30. Smiley C, et al. Evidence-Based Clinical Practice Guideline on the Nonsurgical Treatment of Chronic Periodontal Disease. J Am Dent Assoc 2014;x(x):xx-xx. Sgolastra F, Petrucci A, Severino M, et al. Adjunctive photodynamic therapy to non-surgical treatment of chronic periodontitis: a systematic review and meta-analysis. J Clin Perio 2013;40(5):514-26. Sanz I, Alonso B, Carasol M, Herrera D, Sanz M. Nonsurgical treatment of periodontitis. J Evid Based Dent Pract 2012;12(3 Suppl):76-86. Matesanz-Perez P, Garcia-Gargallo M, Figuero E, et al. A systematic review on the effects of local antimicrobials as adjuncts to subgingival debridement, compared with subgingival debridement alone, in the treatment of chronic periodontitis. J Clin Periodontol 2013;40(3):22741. Herrara D, Matesanz P, Bascones-Martinez A, Sanz M. Local and systemic antimicrobial therapy in periodontics. J Evid Based Dent Pract 2012;S1:50-60. Worthington H, Needleman I. Evidence-based periodontal disease prevention and treatment: introduction. Periodontol 2000 2005;37:9-11. Angaji M, Gelskey S, Nogueira-Filho G, Brothwell D. A systematic review of clinical efficacy of adjunctive antibiotics in the treatment of smokers with periodontitis. J Periodontol 2010;81(11):1518-28. Atieh MA. Photodynamic therapy as an adjunctive treatment for chronic periodontitis: a metaanalysis. Lasers Med Sci 2010;25(4):605-13. Azarpazhooh A, Shah PS, Tenenbaum HC, Goldberg MB. The effect of photodynamic therapy for periodontitis: a systematic review and meta-analysis. J Periodontol 2010;81(1):4-14. Beirne P, Worthington HV, Clarkson JE. Routine scale and polish for periodontal health in adults. Cochrane Database Syst Rev 2007(4):CD004625. Berkey CS, Antczak-Bouckoms A, Hoaglin DC, Mosteller F, Pihlstrom BL. Multiple-outcomes meta-analysis of treatments for periodontal disease. J Dent Res 1995;74(4):1030-9. Cosyn J, Wyn I. A systematic review on the effects of the chlorhexidine chip when used as an adjunct to scaling and root planing in the treatment of chronic periodontitis. J Periodontol 2006;77(2):257-64. Eberhard J, Jepsen S, Jervoe-Storm PM, Needleman I, Worthington HV. Full-mouth disinfection for the treatment of adult chronic periodontitis. Cochrane Database Syst Rev 2008(1):CD004622. Eberhard J, Jervoe-Storm PM, Needleman I, Worthington H, Jepsen S. Full-mouth treatment concepts for chronic periodontitis: a systematic review. J Clin Periodontol 2008;35(7):591-604. Elter JR, Lawrence HP, Offenbacher S, Beck JD. Meta-analysis of the effect of systemic metronidazole as an adjunct to scaling and root planing for adult periodontitis. J Periodontal Res 1997;32(6):487-96. Haffajee AD, Patel M, Socransky SS. Microbiological changes associated with four different periodontal therapies for the treatment of chronic periodontitis. Oral Microbiol Immunol 2008;23(2):148-57. Hallmon WW, Rees TD. Local anti-infective therapy: mechanical and physical approaches. A systematic review. Ann Periodontol 2003;8(1):99-114. Heasman PA, McCracken GI, Steen N. Supportive periodontal care: the effect of periodic subgingival debridement compared with supragingival prophylaxis with respect to clinical outcomes. J Clin Periodontol 2002;29 Suppl 3:163-72; discussion 95-6. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. July 2015 Page 77 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. 39. 40. 41. 42. 43. 44. 45. Hung HC, Douglass CW. Meta-analysis of the effect of scaling and root planing, surgical treatment and antibiotic therapies on periodontal probing depth and attachment loss. J Clin Periodontol 2002;29(11):975-86. Lang NP, Tan WC, Krahenmann MA, Zwahlen M. A systematic review of the effects of full-mouth debridement with and without antiseptics in patients with chronic periodontitis. J Clin Periodontol 2008;35(8 Suppl):8-21. Muller HP. A bivariate multi-level model, which avoids mathematical coupling in the study of change and initial periodontal attachment level after therapy. Clin Oral Investig 2007;11(3):30710. Pavia M, Nobile CG, Bianco A, Angelillo IF. Meta-analysis of local metronidazole in the treatment of chronic periodontitis. J Periodontol 2004;75(6):830-8. Pihlstrom BL, McHugh RB, Oliphant TH, Ortiz-Campos C. Comparison of surgical and nonsurgical treatment of periodontal disease. A review of current studies and additional results after 61/2 years. J Clin Periodontol 1983;10(5):524-41. Sgolastra F, Gatto R, Petrucci A, Monaco A. Effectiveness of systemic amoxicillin/metronidazole as adjunctive therapy to scaling and root planing in the treatment of chronic periodontitis: a systematic review and meta-analysis. J Periodontol 2012;83(10):1257-69. Sgolastra F, Petrucci A, Gatto R, Marzo G, Monaco A. Photodynamic therapy in the treatment of chronic periodontitis: a systematic review and meta-analysis. Lasers Med Sci 2011. Slot DE, Koster TJ, Paraskevas S, Van der Weijden GA. The effect of the Vector scaler system on human teeth: a systematic review. Int J Dent Hyg 2008;6(3):154-65. Slot DE, Kranendonk AA, Paraskevas S, Van der Weijden F. The effect of a pulsed Nd:YAG laser in non-surgical periodontal therapy. J Periodontol 2009;80(7):1041-56. Tunkel J, Heinecke A, Flemmig TF. A systematic review of efficacy of machine-driven and manual subgingival debridement in the treatment of chronic periodontitis. J Clin Periodontol 2002;29 Suppl 3:72-81; discussion 90-1. Van der Weijden GA, Timmerman MF. A systematic review on the clinical efficacy of subgingival debridement in the treatment of chronic periodontitis. J Clin Periodontol 2002;29 Suppl 3:55-71; discussion 90-1. Bonito AJ, Lux L, Lohr KN. Impact of local adjuncts to scaling and root planing in periodontal disease therapy: a systematic review. J Periodontol 2005;76(8):1227-36. Bono A, Brunotto M. Amoxicillin/metronidazole or scaling and root planing in the treatment of chronic periodontitis. Acta Odontol Latinoam 2010;23(3):196-203. Cobb CM. Clinical significance of non-surgical periodontal therapy: an evidence-based perspective of scaling and root planing. J Clin Periodontol 2002;29 Suppl 2:6-16. Cobb CM. Lasers in periodontics: a review of the literature. J Periodontol 2006;77(4):545-64. Hanes PJ, Purvis JP. Local anti-infective therapy: pharmacological agents. A systematic review. Ann Periodontol 2003;8(1):79-98. Herrera D, Sanz M, Jepsen S, Needleman I, Roldan S. A systematic review on the effect of systemic antimicrobials as an adjunct to scaling and root planing in periodontitis patients. J Clin Periodontol 2002;29 Suppl 3:136-59; discussion 60-2. Pavia M, Nobile CG, Angelillo IF. Meta-analysis of local tetracycline in treating chronic periodontitis. J Periodontol 2003;74(6):916-32. Preshaw PM, Hefti AF, Bradshaw MH. Adjunctive subantimicrobial dose doxycycline in smokers and non-smokers with chronic periodontitis. J Clin Periodontol 2005;32(6):610-6. Reddy MS, Geurs NC, Gunsolley JC. Periodontal host modulation with antiproteinase, antiinflammatory, and bone-sparing agents. A systematic review. Ann Periodontol 2003;8(1):12-37. Sahrmann P, Puhan MA, Attin T, Schmidlin PR. Systematic review on the effect of rinsing with povidone-iodine during nonsurgical periodontal therapy. J Periodontal Res 2010;45(2):153-64. Sgolastra F, Petrucci A, Gatto R, Giannoni M, Monaco A. Long-term efficacy of subantimicrobialdose doxycycline as an adjunctive treatment to scaling and root planing: a systematic review and meta-analysis. J Periodontol 2011;82(11):1570-81. Zandbergen D, Slot DE, Cobb CM, Van der Weijden FA. The Clinical Effect of Scaling and Root Planing and The Concomitant Administration of Systemic Amoxicillin and Metronidazole: A Systematic Review. J Periodontol 2012. July 2015 Page 78 46. 47. 48. 49. 50. 51. 52. 53. 54. 55. 56. 57. 58. 59. 60. 61. 62. 63. 64. 65. 66. Smail-Faugeron V, Fron-Chabouis H, Courson F, Durieux P. Comparison of intervention effects in split-mouth and parallel-arm randomized controlled trials: a meta-epidemiological study. BMC Med Res Methodol 2014;14:64. U. S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Guidance for Industry - Gingivitis: Development and Evaluation of Drugs for Treatment or Prevention (Draft). Available at: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm07 1693.pdf; accessed January 13, 2014.; 2005. Reddy MS, Palcanis KG, Geurs NC. A comparison of manual and controlled-force attachmentlevel measurements. J Clin Periodontol 1997;24(12):920-6. Worthington HV, Clarkson JE, Bryan G, Beirne PV. Routine scale and polish for periodontal health in adults. Cochrane Database of Systematic Reviews: John Wiley & Sons, Ltd; 2013. Higgins JPT, Altman DG, Sterne JAC, Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 The Cochrane Collaboration; updated March 2011. Cobb CM. Non-surgical pocket therapy: mechanical. Ann Periodontol 1996;1(1):443-90. Imrey PB, Chilton NW, Pihlstrom B, et al. Proposed guidelines for American Dental Association acceptance of products for professional, non-surgical treatment of adult periodontitis: Task Force on Design and Analysis of Dental and Oral Research. J Periodontal Res 1994;29(5):348-60. Ronderos M, Michalowicz BS. Epidemiology of periodontal diseases and risk factors. In: Rose LF, Mealey BL, Genco RJ, Cohen DW, editors. Periodontics: Medicine, Surgery and Implants. Philadelphia, PA: Elsevier Mosby; 2004. p. 39, 302. Armitage G. Manual periodontal probing in supportive periodontal therapy. Periodontol 2000 1996;12:33-39. Higgins JPT, Green S, (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0: The Cochrane Collaboration. Available from www.cochrane-handbook.org; updated March 2011. Cochrane Cystic Fibrosis and Genetic Disorders Group. The generic inverse variance method. Available at: http://cfgd.cochrane.org/sites/cfgd.cochrane.org/files/uploads/Generic%20Inverse%20Variance% 20method.doc; updated 7 Feb 2013. The Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager (RevMan) [Computer program]. Version 5.2. Copenhagen; 2012. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Syn Meth 2010;1:97-111. Riley RD, Higgins JPT, Deeks JJ. Interpretation of random effects meta-analysis. BMJ 2011;342:964-7. Needleman IG, Worthington H, Giedrys-Leeper E, Tucker R. Guided tissue regeneration for periodontal infra-bony defects. Cochrane Database of Systematic Reviews 2006;Apr 19(2):CD001724. Preshaw PM, Hefti AF, Novak MJ, et al. Subantimicrobial dose doxycycline enhances the efficacy of scaling and root planing in chronic periodontitis: a multicenter trial. J Periodontol 2004;75(8):1068-76. Mohammad AR, Preshaw PM, Bradshaw MH, et al. Adjunctive subantimicrobial dose doxycycline in the management of institutionalised geriatric patients with chronic periodontitis. Gerodontology 2005;22(1):37-43. American Dental Association. ADA Clinical Practice Guideline Handbook - 2013 Update. Chicago, IL. Takkouche B, Cadarso-Suarez C, Spiegelman D. Evaluation of old and new tests of heterogeneity in epidemiologic meta-analysis. Am J Epidemiol 1999;150(2):206-15. Huedo-Medina T, Sanchez-Meca J, Marin-Martinez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index?". CHIP Documents, Paper 19 available at http://digitalcommons.uconn.edu/chip_docs/19 2006. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence inconsistency. J Clin Epidemiol 2011;64:1294-302. July 2015 Page 79 67. 68. 69. 70. 71. 72. 73. 74. 75. 76. 77. 78. 79. 80. 81. 82. 83. 84. 85. 86. 87. 88. 89. Ioannidis JPA. Interpretation of tests of heterogeneity and bias in meta-analysis. J Eval Clin Pract 2008;14:951-7. StataCorp. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP; 2011. Feng HS, Bernardo CC, Sonoda LL, et al. Subgingival ultrasonic instrumentation of residual pockets irrigated with essential oils: a randomized controlled trial. J Clin Periodontol 2011;38(7):637-43. Schlagenhauf U, Stellwag P, Fiedler A. Subgingival irrigation in the maintenance phase of periodontal therapy. J Clin Periodontol 1990;17(9):650-3. Wennstrom JL, Heijl L, Dahlen G, Grondahl K. Periodic subgingival antimicrobial irrigation of periodontal pockets (I). Clinical observations. J Clin Periodontol 1987;14(9):541-50. Del Peloso Ribeiro E, Bittencourt S, Ambrosano GM, et al. Povidone-iodine used as an adjunct to non-surgical treatment of furcation involvements. J Periodontol 2006;77(2):211-7. Ribeiro Edel P, Bittencourt S, Sallum EA, et al. Non-surgical instrumentation associated with povidone-iodine in the treatment of interproximal furcation involvements. J Appl Oral Sci 2010;18(6):599-606. Rosling B, Hellstrom MK, Ramberg P, Socransky SS, Lindhe J. The use of PVP-iodine as an adjunct to non-surgical treatment of chronic periodontitis. J Clin Periodontol 2001;28(11):1023-31. Braatz L, Garrett S, Claffey N, Egelberg J. Antimicrobial irrigation of deep pockets to supplement non-surgical periodontal therapy. II. Daily irrigation. J Clin Periodontol 1985;12(8):630-8. Leonhardt A, Bergstrom C, Krok L, Cardaropoli G. Healing following ultrasonic debridement and PVP-iodine in individuals with severe chronic periodontal disease: a randomized, controlled clinical study. Acta Odontol Scand 2006;64(5):262-6. MacAlpine R, Magnusson I, Kiger R, et al. Antimicrobial irrigation of deep pockets to supplement oral hygiene instruction and root debridement. I. Bi-weekly irrigation. J Clin Periodontol 1985;12(7):568-77. Berglundh T, Krok L, Liljenberg B, et al. The use of metronidazole and amoxicillin in the treatment of advanced periodontal disease. A prospective, controlled clinical trial. J Clin Periodontol 1998;25(5):354-62. Kahl M, Haase E, Kocher T, Ruhling A. Clinical effects after subgingival polishing with a nonaggressive ultrasonic device in initial therapy. J Clin Periodontol 2007;34(4):318-24. Lindhe J, Liljenberg B, Adielsson B. Effect of long-term tetracycline therapy on human periodontal disease. J Clin Periodontol 1983;10(6):590-601. Neill ME, Mellonig JT. Clinical efficacy of the Nd:YAG laser for combination periodontitis therapy. Pract Periodontics Aesthet Dent 1997;9(6 Suppl):1-5. Ng VW, Bissada NF. Clinical evaluation of systemic doxycycline and ibuprofen administration as an adjunctive treatment for adult periodontitis. J Periodontol 1998;69(7):772-6. Rotundo R, Nieri M, Cairo F, et al. Lack of adjunctive benefit of Er:YAG laser in non-surgical periodontal treatment: a randomized split-mouth clinical trial. J Clin Periodontol 2010;37(6):52633. Chen L, Luo G, Xuan D, et al. Effects of non-surgical periodontal treatment on clinical response, serum inflammatory parameters, and metabolic control in patients with type 2 diabetes: a randomized study. J Periodontol 2012;83(4):435-43. Jones AA, Kornman KS, Newbold DA, Manwell MA. Clinical and microbiological effects of controlled-release locally delivered minocycline in periodontitis. J Periodontol 1994;65(11):105866. Ribeiro IW, Sbrana MC, Esper LA, Almeida AL. Evaluation of the effect of the GaAlAs laser on subgingival scaling and root planing. Photomed Laser Surg 2008;26(4):387-91. Van Dyke TE, Offenbacher S, Braswell L, Lessem J. Enhancing the value of scaling and rootplaning: Arestin clinical trial results. J Int Acad Periodontol 2002;4(3):72-6. Zhou X, Han J, Liu Z, et al. Effects of periodontal treatment on lung function and exacerbation frequency in patients with chronic obstructive pulmonary disease and chronic periodontitis: a 2year pilot randomized controlled trial. J Clin Periodontol 2014;41(6):564-72. Ide M, Jagdev D, Coward PY, et al. The short-term effects of treatment of chronic periodontitis on circulating levels of endotoxin, C-reactive protein, tumor necrosis factor-alpha, and interleukin-6. J Periodontol 2004;75(3):420-8. July 2015 Page 80 90. 91. 92. 93. 94. 95. 96. 97. 98. 99. 100. 101. 102. 103. 104. 105. 106. 107. 108. 109. 110. Gu Y, Walker C, Ryan ME, Payne JB, Golub LM. Non-antibacterial tetracycline formulations: clinical applications in dentistry and medicine. J Oral Microbiol 2012;4:19227 Al Mubarak S, Abou Rass M, Alsuwyed A, et al. A new paradigm between mechanical scaling and root planing combined with adjunctive chemotherapy for glycated hemoglobin improvement in diabetics. Intl J Diabetes Mellit 2010;2(3):158-64. Caton JG, Ciancio SG, Blieden TM, et al. Treatment with subantimicrobial dose doxycycline improves the efficacy of scaling and root planing in patients with adult periodontitis. J Periodontol 2000;71(4):521-32. Deo V, Gupta S, Bhongade ML, Jaiswal R. Evaluation of subantimicrobial dose doxycycline as an adjunct to scaling and root planing in chronic periodontitis patients with diabetes: a randomized, placebo-controlled clinical trial. J Contemp Dent Pract 2010;11(3):009-16. Emingil G, Atilla G, Sorsa T, et al. The effect of adjunctive low-dose doxycycline therapy on clinical parameters and gingival crevicular fluid matrix metalloproteinase-8 levels in chronic periodontitis. J Periodontol 2004;75(1):106-15. Emingil G, Atilla G, Sorsa T, Savolainen P, Baylas H. Effectiveness of adjunctive low-dose doxycycline therapy on clinical parameters and gingival crevicular fluid laminin-5 gamma2 chain levels in chronic periodontitis. J Periodontol 2004;75(10):1387-96. Emingil G, Atilla G, Sorsa T, Tervahartiala T. The effect of adjunctive subantimicrobial dose doxycycline therapy on GCF EMMPRIN levels in chronic periodontitis. J Periodontol 2008;79(3):469-76. Emingil G, Gurkan A, Atilla G, Kantarci A. Subantimicrobial-dose doxycycline and cytokinechemokine levels in gingival crevicular fluid. J Periodontol 2011;82(3):452-61. Gurkan A, Emingil G, Cinarcik S, Berdeli A. Post-treatment effects of subantimicrobial dose doxycycline on clinical parameters and gingival crevicular fluid transforming growth factor-beta1 in severe, generalized chronic periodontitis. Int J Dent Hyg 2008;6(2):84-92. Haffajee AD, Torresyap G, Socransky SS. Clinical changes following four different periodontal therapies for the treatment of chronic periodontitis: 1-year results. J Clin Periodontol 2007;34(3):243-53. Needleman I, Suvan J, Gilthorpe MS, et al. A randomized-controlled trial of low-dose doxycycline for periodontitis in smokers. J Clin Periodontol 2007;34(4):325-33. Preshaw PM, Novak MJ, Mellonig J, et al. Modified-release subantimicrobial dose doxycycline enhances scaling and root planing in subjects with periodontal disease. J Periodontol 2008;79(3):440-52. El-Shinnawi UM, El-Tantawy SI. The effect of alendronate sodium on alveolar bone loss in periodontitis (clinical trial). J Int Acad Periodontol 2003;5(1):5-10. Lane N, Armitage GC, Loomer P, et al. Bisphosphonate therapy improves the outcome of conventional periodontal treatment: results of a 12-month, randomized, placebo-controlled study. J Periodontol 2005;76(7):1113-22. El-Sharkawy H, Aboelsaad N, Eliwa M, et al. Adjunctive treatment of chronic periodontitis with daily dietary supplementation with omega-3 Fatty acids and low-dose aspirin. J Periodontol 2010;81(11):1635-43. Reddy MS, Palcanis KG, Barnett ML, et al. Efficacy of meclofenamate sodium (Meclomen) in the treatment of rapidly progressive periodontitis. J Clin Periodontol 1993;20(9):635-40. Yen CA, Damoulis PD, Stark PC, et al. The effect of a selective cyclooxygenase-2 inhibitor (celecoxib) on chronic periodontitis. J Periodontol 2008;79(1):104-13. Preshaw PM, Hefti AF, Jepsen S, et al. Subantimicrobial dose doxycycline as adjunctive treatment for periodontitis. A review. J Clin Periodontol 2004;31(9):697-707. FDA Approved Drug Products. Periostat (doxycycline hyclate tablets) 20 mg label information. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2004/50783slr002_periostat_lbl.pdf. Accessed January 14, 2014. 2004. Al-Joburi W, Quee TC, Lautar C, et al. Effects of adjunctive treatment of periodontitis with tetracycline and spiramycin. J Periodontol 1989;60(10):533-9. Cionca N, Giannopoulou C, Ugolotti G, Mombelli A. Amoxicillin and metronidazole as an adjunct to full-mouth scaling and root planing of chronic periodontitis. J Periodontol 2009;80(3):364-71. July 2015 Page 81 111. 112. 113. 114. 115. 116. 117. 118. 119. 120. 121. 122. 123. 124. 125. 126. 127. 128. 129. 130. Flemmig TF, Milian E, Karch H, Klaiber B. Differential clinical treatment outcome after systemic metronidazole and amoxicillin in patients harboring Actinobacillus actinomycetemcomitans and/or Porphyromonas gingivalis. J Clin Periodontol 1998;25(5):380-7. Gomi K, Yashima A, Nagano T, et al. Effects of full-mouth scaling and root planing in conjunction with systemically administered azithromycin. J Periodontol 2007;78(3):422-9. Goodson JM, Haffajee AD, Socransky SS, et al. Control of periodontal infections: a randomized controlled trial I. The primary outcome attachment gain and pocket depth reduction at treated sites. J Clin Periodontol 2012;39(6):526-36. Guentsch A, Jentsch H, Pfister W, Hoffmann T, Eick S. Moxifloxacin as an adjunctive antibiotic in the treatment of severe chronic periodontitis. J Periodontol 2008;79(10):1894-903. Mascarenhas P, Gapski R, Al-Shammari K, et al. Clinical response of azithromycin as an adjunct to non-surgical periodontal therapy in smokers. J Periodontol 2005;76(3):426-36. Mombelli A, Brochut P, Plagnat D, Casagni F, Giannopoulou C. Enamel matrix proteins and systemic antibiotics as adjuncts to non-surgical periodontal treatment: clinical effects. J Clin Periodontol 2005;32(3):225-30. Oteo A, Herrera D, Figuero E, et al. Azithromycin as an adjunct to scaling and root planing in the treatment of Porphyromonas gingivalis-associated periodontitis: a pilot study. J Clin Periodontol 2010;37(11):1005-15. Palmer RM, Matthews JP, Wilson RF. Non-surgical periodontal treatment with and without adjunctive metronidazole in smokers and non-smokers. J Clin Periodontol 1999;26(3):158-63. Pradeep AR, Kathariya R. Clarithromycin, as an adjunct to non surgical periodontal therapy for chronic periodontitis: a double blinded, placebo controlled, randomized clinical trial. Arch Oral Biol 2011;56(10):1112-9. Ramberg P, Rosling B, Serino G, et al. The long-term effect of systemic tetracycline used as an adjunct to non-surgical treatment of advanced periodontitis. J Clin Periodontol 2001;28(5):446-52. Ribeiro Edel P, Bittencourt S, Zanin IC, et al. Full-mouth ultrasonic debridement associated with amoxicillin and metronidazole in the treatment of severe chronic periodontitis. J Periodontol 2009;80(8):1254-64. Sampaio E, Rocha M, Figueiredo LC, et al. Clinical and microbiological effects of azithromycin in the treatment of generalized chronic periodontitis: a randomized placebo-controlled clinical trial. J Clin Periodontol 2011;38(9):838-46. Yashima A, Gomi K, Maeda N, Arai T. One-stage full-mouth versus partial-mouth scaling and root planing during the effective half-life of systemically administered azithromycin. J Periodontol 2009;80(9):1406-13. Han B, Emingil G, Ozdemir G, et al. Azithromycin as an adjunctive treatment of generalized severe chronic periodontitis: clinical, microbiologic, and biochemical parameters. J Periodontol 2012;83(12):1480-91. Martande SS, Pradeep AR, Singh SP, et al. Clinical and microbiological effects of systemic azithromycin in adjunct to nonsurgical periodontal therapy in treatment of Aggregatibacter actinomycetemcomitans associated periodontitis: a randomized placebo-controlled clinical trial. J Investig Clin Dent 2014. Miranda TS, Feres M, Perez-Chaparro PJ, et al. Metronidazole and amoxicillin as adjuncts to scaling and root planing for the treatment of type 2 diabetic subjects with periodontitis: 1-year outcomes of a randomized placebo-controlled clinical trial. J Clin Periodontol 2014. Preus HR, Gunleiksrud TM, Sandvik L, Gjermo P, Baelum V. A randomized, double-masked clinical trial comparing four periodontitis treatment strategies: 1-year clinical results. J Periodontol 2013;84(8):1075-86. Tsalikis L, Sakellari D, Dagkalis P, Boura P, Konstantinidis A. Effects of doxycycline on clinical, microbiological and immunological parameters in well-controlled diabetes type- 2 patients with periodontal disease. A randomized controlled clinical trial. J Clin Periodontol 2014. Center for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013: U. S. Department of Health and Human Services. Eickholz P, Kim TS, Schacher B, et al. Subgingival topical doxycycline versus mechanical debridement for supportive periodontal therapy: a single blind randomized controlled two-center study. Am J Dent 2005;18(6):341-6. July 2015 Page 82 131. 132. 133. 134. 135. 136. 137. 138. 139. 140. 141. 142. 143. 144. 145. 146. 147. 148. 149. 150. Ryder MI, Pons B, Adams D, et al. Effects of smoking on local delivery of controlled-release doxycycline as compared to scaling and root planing. J Clin Periodontol 1999;26(10):683-91. Garrett S, Johnson L, Drisko CH, et al. Two multi-center studies evaluating locally delivered doxycycline hyclate, placebo control, oral hygiene, and scaling and root planing in the treatment of periodontitis. J Periodontol 1999;70(5):490-503. Aimetti M, Romano F, Torta I, et al. Debridement and local application of tetracycline-loaded fibres in the management of persistent periodontitis: results after 12 months. J Clin Periodontol 2004;31(3):166-72. Drisko CL, Cobb CM, Killoy WJ, et al. Evaluation of periodontal treatments using controlledrelease tetracycline fibers: clinical response. J Periodontol 1995;66(8):692-9. Friesen LR, Williams KB, Krause LS, Killoy WJ. Controlled local delivery of tetracycline with polymer strips in the treatment of periodontitis. J Periodontol 2002;73(1):13-9. Maze GI, Reinhardt RA, Agarwal RK, et al. Response to intracrevicular controlled delivery of 25% tetracycline from poly(lactide/glycolide) film strips in SPT patients. J Clin Periodontol 1995;22(11):860-7. Michalowicz BS, Pihlstrom BL, Drisko CL, et al. Evaluation of periodontal treatments using controlled-release tetracycline fibers: Maintenance response. J Periodontol 1995;66(8):708-15. Mombelli A, Lehmann B, Tonetti M, Lang NP. Clinical response to local delivery of tetracycline in relation to overall and local periodontal conditions. J Clin Periodontol 1997;24(7):470-7. Newman MG, Kornman KS, Doherty FM. A 6-month multi-center evaluation of adjunctive tetracycline fiber therapy used in conjunction with scaling and root planing in maintenance patients: clinical results. J Periodontol 1994;65(7):685-91. Romano F, Torta I, Debernardi C, Aimetti M. Debridement and local application of tetracycline in the management of persistent periodontitis. Clinical and microbiological results after 12 months. Minerva Stomatol 2005;54(1-2):43-51. Tonetti MS, Cortellini P, Carnevale G, et al. A controlled multicenter study of adjunctive use of tetracycline periodontal fibers in mandibular class II furcations with persistent bleeding. J Clin Periodontol 1998;25(9):728-36. Wilson TG, Jr., McGuire MK, Greenstein G, Nunn M. Tetracycline fibers plus scaling and root planing versus scaling and root planing alone: Similar results after 5 years. J Periodontol 1997;68(11):1029-32. Wong MY, Lu CL, Liu CM, Hou LT, Chang WK. Clinical response of localized recurrent periodontitis treated with scaling, root planing, and tetracycline fiber. J Formos Med Assoc 1998;97(7):490-7. Jain R, Mohamed F, Hemalatha M. Minocycline containing local drug delivery system in the management of chronic periodontitis: A randomized controlled trial. J Indian Soc Periodontol 2012;16(2):179-83. Lin SJ, Tu YK, Tsai SC, Lai SM, Lu HK. Non-surgical periodontal therapy with and without subgingival minocycline administration in patients with poorly controlled type II diabetes: a randomized controlled clinical trial. Clin Oral Investig 2012;16(2):599-609. Timmerman MF, van der Weijden GA, van Steenbergen TJ, et al. Evaluation of the long-term efficacy and safety of locally-applied minocycline in adult periodontitis patients. J Clin Periodontol 1996;23(8):707-16. van Steenberghe D, Rosling B, Soder PO, et al. A 15-month evaluation of the effects of repeated subgingival minocycline in chronic adult periodontitis. J Periodontol 1999;70(6):657-67. Buduneli E, Tunger A, Evrenosoglu E, Bilgic A. Comparative clinical and microbiological effects of subgingival metronidazole application in adult periodontitis; 12-months results. J Int Acad Periodontol 2001;3(4):81-6. Griffiths GS, Smart GJ, Bulman JS, et al. Comparison of clinical outcomes following treatment of chronic adult periodontitis with subgingival scaling or subgingival scaling plus metronidazole gel. J Clin Periodontol 2000;27(12):910-7. Knofler G, Purschwitz R, Jentsch H, Birkenmeier G, Schmidt H. Gingival crevicular fluid levels of aspartate aminotransferase and alpha2-macroglobulin before and after topical application of metronidazole or scaling and root planing. Quintessence Int 2008;39(5):381-9. July 2015 Page 83 151. 152. 153. 154. 155. 156. 157. 158. 159. 160. 161. 162. 163. 164. 165. 166. 167. 168. 169. 170. Leiknes T, Leknes KN, Boe OE, Skavland RJ, Lie T. Topical use of a metronidazole gel in the treatment of sites with symptoms of recurring chronic inflammation. J Periodontol 2007;78(8):1538-44. Lie T, Bruun G, Boe OE. Effects of topical metronidazole and tetracycline in treatment of adult periodontitis. J Periodontol 1998;69(7):819-27. Pedrazzoli V, Kilian M, Karring T. Comparative clinical and microbiological effects of topical subgingival application of metronidazole 25% dental gel and scaling in the treatment of adult periodontitis. J Clin Periodontol 1992;19(9 Pt 2):715-22. Rudhart A, Purucker P, Kage A, Hopfenmuller W, Bernimoulin JP. Local metronidazole application in maintenance patients. Clinical and microbiological evaluation. J Periodontol 1998;69(10):1148-54. Stelzel M, Flores-de-Jacoby L. Topical metronidazole application as an adjunct to scaling and root planing. J Clin Periodontol 2000;27(6):447-52. Agarwal E, Pradeep AR, Bajaj P, Naik SB. Efficacy of local drug delivery of 0.5% clarithromycin gel as an adjunct to non-surgical periodontal therapy in the treatment of current smokers with chronic periodontitis: a randomized controlled clinical trial. J Periodontol 2012;83(9):1155-63. Bajaj P, Pradeep AR, Agarwal E, Kumari M, Naik SB. Locally delivered 0.5% clarithromycin, as an adjunct to nonsurgical treatment in chronic periodontitis with well-controlled type 2 diabetes: a randomized controlled clinical trial. J Investig Clin Dent 2012. Agarwal E, Bajaj P, Naik SB, Pradeep AR. Locally Delivered 0.5% Azithromycin, as an Adjunct to Non Surgical Treatment in Chronic Periodontitis With Type 2 Diabetes: A Randomized Controlled Clinical Trial. J Periodontol 2012. Cosyn J, Sabzevar MM. Subgingival chlorhexidine varnish administration as an adjunct to sameday full-mouth root planing. II. Microbiological observations. J Periodontol 2007;78(3):438-45. Cosyn J, Wyn I, De Rouck T, Sabzevar MM. Clinical benefits of subgingival chlorhexidine varnish application as an adjunct to same-day full-mouth root planing: a pilot study. J Periodontol 2006;77(6):1074-9. Cosyn J, Wyn I, De Rouck T, Sabzevar MM. Long-term clinical effects of a chlorhexidine varnish implemented treatment strategy for chronic periodontitis. J Periodontol 2006;77(3):406-15. Knofler GU, Purschwitz RE, Eick S, et al. Microbiologic findings 1 year after partial- and fullmouth scaling in the treatment of moderate chronic periodontitis. Quintessence Int 2011;42(9):e107-17. Matesanz P, Herrera D, Echeverria A, et al. A randomized clinical trial on the clinical and microbiological efficacy of a xanthan gel with chlorhexidine for subgingival use. Clin Oral Investig 2012. Mongardini C, van Steenberghe D, Dekeyser C, Quirynen M. One stage full- versus partial-mouth disinfection in the treatment of chronic adult or generalized early-onset periodontitis. I. Long-term clinical observations. J Periodontol 1999;70(6):632-45. Paolantonio M, D'Ercole S, Pilloni A, et al. Clinical, microbiologic, and biochemical effects of subgingival administration of a Xanthan-based chlorhexidine gel in the treatment of periodontitis: a randomized multicenter trial. J Periodontol 2009;80(9):1479-92. Perinetti G, Paolantonio M, Cordella C, et al. Clinical and microbiological effects of subgingival administration of two active gels on persistent pockets of chronic periodontitis patients. J Clin Periodontol 2004;31(4):273-81. Blomlof L, Bergman E, Forsgardh A, et al. A clinical study of root surface conditioning with an EDTA gel. I. Nonsurgical periodontal treatment. Int J Periodontics Restorative Dent 2000;20(6):560-5. Akncbay H, Senel S, Ay ZY. Application of chitosan gel in the treatment of chronic periodontitis. J Biomed Mater Res B Appl Biomater 2007;80(2):290-6. Azmak N, Atilla G, Luoto H, Sorsa T. The effect of subgingival controlled-release delivery of chlorhexidine chip on clinical parameters and matrix metalloproteinase-8 levels in gingival crevicular fluid. J Periodontol 2002;73(6):608-15. Gonzales JR, Harnack L, Schmitt-Corsitto G, et al. A novel approach to the use of subgingival controlled-release chlorhexidine delivery in chronic periodontitis: a randomized clinical trial. J Periodontol 2011;82(8):1131-9. July 2015 Page 84 171. 172. 173. 174. 175. 176. 177. 178. 179. 180. 181. 182. 183. 184. 185. 186. 187. 188. 189. 190. Heasman PA, Heasman L, Stacey F, McCracken GI. Local delivery of chlorhexidine gluconate (PerioChip) in periodontal maintenance patients. J Clin Periodontol 2001;28(1):90-5. Paolantonio M, D'Angelo M, Grassi RF, et al. Clinical and microbiologic effects of subgingival controlled-release delivery of chlorhexidine chip in the treatment of periodontitis: a multicenter study. J Periodontol 2008;79(2):271-82. Paolantonio M, Dolci M, Perfetti G, et al. Effect of a subgingival chlorhexidine chip on the clinical parameters and the levels of alkaline phosphatase activity in gingival crevicular fluid during the non-surgical treatment of periodontitis. J Biol Regul Homeost Agents 2008;22(1):63-72. Sakellari D, Ioannidis I, Antoniadou M, Slini T, Konstantinidis A. Clinical and microbiological effects of adjunctive, locally delivered chlorhexidine on patients with chronic periodontitis. J Int Acad Periodontol 2010;12(1):20-6. Dimitra S, Loannis L, Malama A, Theodora S, Antonis K. Clinical and microbiological effects of adjunctive, locally delivered chlorhexidine on patients with chronic periodontitis. J Int Acad Periodontol 2010;12(1):20-26. Grisi DC, Salvador SL, Figueiredo LC, et al. Effect of a controlled-release chlorhexidine chip on clinical and microbiological parameters of periodontal syndrome. J Clin Periodontol 2002;29(10):875-81. Jeffcoat MK, Bray KS, Ciancio SG, et al. Adjunctive use of a subgingival controlled-release chlorhexidine chip reduces probing depth and improves attachment level compared with scaling and root planing alone. J Periodontol 1998;69(9):989-97. Jeffcoat MK, Palcanis KG, Weatherford TW, et al. Use of a biodegradable chlorhexidine chip in the treatment of adult periodontitis: clinical and radiographic findings. J Periodontol 2000;71(2):256-62. Mizrak T, Guncu GN, Caglayan F, et al. Effect of a controlled-release chlorhexidine chip on clinical and microbiological parameters and prostaglandin E2 levels in gingival crevicular fluid. J Periodontol 2006;77(3):437-43. Soskolne WA, Heasman PA, Stabholz A, et al. Sustained local delivery of chlorhexidine in the treatment of periodontitis: a multi-center study. J Periodontol 1997;68(1):32-8. Rodrigues IF, Machion L, Casati MZ, et al. Clinical evaluation of the use of locally delivered chlorhexidine in periodontal maintenance therapy. J Periodontol 2007;78(4):624-8. FDA U. S. Food and Drug Administration Safety Information - PerioChip (chlorhexidine gluconate); available at http://www.fda.gov/safety/medwatch/safetyinformation/ucm330906.htm. 2012. Accessed 12 30 2013. Agan S, Sonmez S, Serdar M. The effect of topical doxycycline usage on gingival crevicular fluid MMP-8 levels of chronic and aggressive periodontitis patients: a pilot study. Int J Dent Hyg 2006;4(3):114-21. Machion L, Andia DC, Benatti BB, et al. Locally delivered doxycycline as an adjunctive therapy to scaling and root planing in the treatment of smokers: a clinical study. J Periodontol 2004;75(3):464-9. Martorelli de Lima AF, Cury CC, Palioto DB, et al. Therapy with adjunctive doxycycline local delivery in patients with type 1 diabetes mellitus and periodontitis. J Clin Periodontol 2004;31(8):648-53. Machion L, Andia DC, Lecio G, et al. Locally delivered doxycycline as an adjunctive therapy to scaling and root planing in the treatment of smokers: a 2-year follow-up. J Periodontol 2006;77(4):606-13. Tonetti MS, Lang NP, Cortellini P, et al. Effects of a single topical doxycycline administration adjunctive to mechanical debridement in patients with persistent/recurrent periodontitis but acceptable oral hygiene during supportive periodontal therapy. J Clin Periodontol 2012;39(5):47582. Oringer RJ, Van Dyke TE, Lessem J. The challenge of treating periodontal patients who smoke-the efficacy of Arestin. J Int Acad Periodontol 2002;4(3):89-94. FDA Center for Drug Application Reviews. Statistical Review and Evaluation, Application Number NDA 50-781. available at: www.fda.gov; 2000. Wennstrom JL, Newman HN, MacNeill SR, et al. Utilisation of locally delivered doxycycline in non-surgical treatment of chronic periodontitis. A comparative multi-centre trial of 2 treatment approaches. J Clin Periodontol 2001;28(8):753-61. July 2015 Page 85 191. 192. 193. 194. 195. 196. 197. 198. 199. 200. 201. 202. 203. 204. 205. 206. 207. 208. 209. 210. Garrett S. Local delivery of doxycycline for the treatment of periodontitis. Compend Contin Educ Dent 1999;20(5):437-40, 42, 44 passim; quiz 48. FDA Approved Drug Products. Atridox (doxycycline hyclate) 10% label information. Available at: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails. Accessed on January 14, 2014.; 2011. Henderson RJ, Boyens JV, Holborow DW, Pack AR. Scaling and root-planing treatment with adjunctive subgingival minocycline. A clinical pilot study over six months, of sites adjacent to and remote from the antibiotic application. J Int Acad Periodontol 2002;4(3):77-87. Skaleric U, Schara R, Medvescek M, et al. Periodontal treatment by Arestin and its effects on glycemic control in type 1 diabetes patients. J Int Acad Periodontol 2004;6(4 Suppl):160-5. Williams RC, Paquette DW, Offenbacher S, et al. Treatment of periodontitis by local administration of minocycline microspheres: a controlled trial. J Periodontol 2001;72(11):1535-44. FDA Approved Drug Products. Arestin (minocycline hydrochloride) Microspheres, 1 mg label information. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/050781s019lbl.pdf. Accessed on January 14, 2014. 2012. Dilsiz A, Canakci V, Aydin T. Clinical Effects of KTP Laser and Photodynamic Therapy on Outcomes of Treatment of Chronic Periodontitis: A Randomized Controlled Clinical Trial. J Periodontol 2012. Berakdar M, Callaway A, Eddin MF, Ross A, Willershausen B. Comparison between scaling-rootplaning (SRP) and SRP/photodynamic therapy: six-month study. Head Face Med 2012;8:12. Chondros P, Nikolidakis D, Christodoulides N, et al. Photodynamic therapy as adjunct to nonsurgical periodontal treatment in patients on periodontal maintenance: a randomized controlled clinical trial. Lasers Med Sci 2009;24(5):681-8. Christodoulides N, Nikolidakis D, Chondros P, et al. Photodynamic therapy as an adjunct to nonsurgical periodontal treatment: a randomized, controlled clinical trial. J Periodontol 2008;79(9):1638-44. Giannelli M, Formigli L, Lorenzini L, Bani D. Combined photoablative and photodynamic diode laser therapy as an adjunct to non-surgical periodontal treatment. A randomized split-mouth clinical trial. J Clin Periodontol 2012;39(10):962-70. Theodoro LH, Silva SP, Pires JR, et al. Clinical and microbiological effects of photodynamic therapy associated with nonsurgical periodontal treatment. A 6-month follow-up. Lasers Med Sci 2012;27(4):687-93. Filho GAN, Casarin RC, Casati MZ, Giovani EM. PDT in non-surgical treatment of periodontitis in HIV patients: a split-mouth, randomized clinical trial. Lasers Surg Med 2012;44(4):296-302. Alwaeli HA, Al-Khateeb SN, Al-Sadi A. Long-term clinical effect of adjunctive antimicrobial photodynamic therapy in periodontal treatment: a randomized clinical trial. Lasers Med Sci 2013. Betsy J, Prasanth CS, Baiju KV, Prasanthila J, Subhash N. Efficacy of antimicrobial photodynamic therapy in the management of chronic periodontitis: a randomized controlled clinical trial. J Clin Periodontol 2014;41(6):573-81. Dilsiz A, Canakci V, Aydin T. Clinical effects of potassium-titanyl-phosphate laser and photodynamic therapy on outcomes of treatment of chronic periodontitis: a randomized controlled clinical trial. J Periodontol 2013;84(3):278-86. Luchesi VH, Pimentel SP, Kolbe MF, et al. Photodynamic therapy in the treatment of class II furcation: a randomized controlled clinical trial. J Clin Periodontol 2013;40(8):781-8. Euzebio Alves VT, de Andrade AK, Toaliar JM, et al. Clinical and microbiological evaluation of high intensity diode laser adjutant to non-surgical periodontal treatment: a 6-month clinical trial. Clin Oral Investig 2013;17(1):87-95. Saglam M, Kantarci A, Dundar N, Hakki SS. Clinical and biochemical effects of diode laser as an adjunct to nonsurgical treatment of chronic periodontitis: a randomized, controlled clinical trial. Lasers Med Sci 2014;29(1):37-46. Ustun K, Erciyas K, Sezer U, et al. Clinical and biochemical effects of 810 nm diode laser as an adjunct to periodontal therapy: a randomized split-mouth clinical trial. Photomed Laser Surg 2014;32(2):61-6. July 2015 Page 86 211. 212. 213. 214. 215. 216. 217. 218. Caruso U, Nastri L, Piccolomini R, et al. Use of diode laser 980 nm as adjunctive therapy in the treatment of chronic periodontitis. A randomized controlled clinical trial. New Microbiol 2008;31(4):513-8. Eltas A, Orbak R. Effect of 1,064-nm Nd:YAG laser therapy on GCF IL-1beta and MMP-8 levels in patients with chronic periodontitis. Lasers Med Sci 2012;27(3):543-50. Eltas A, Orbak R. Clinical effects of Nd:YAG laser applications during nonsurgical periodontal treatment in smoking and nonsmoking patients with chronic periodontitis. Photomed Laser Surg 2012;30(7):360-6. Kelbauskiene S, Baseviciene N, Goharkhay K, Moritz A, Machiulskiene V. One-year clinical results of Er,Cr:YSGG laser application in addition to scaling and root planing in patients with early to moderate periodontitis. Lasers Med Sci 2011;26(4):445-52. Lopes BM, Theodoro LH, Melo RF, Thompson GM, Marcantonio RA. Clinical and microbiologic follow-up evaluations after non-surgical periodontal treatment with erbium:YAG laser and scaling and root planing. J Periodontol 2010;81(5):682-91. Garrett S, Adams DF, Bogle G, et al. The effect of locally delivered controlled-release doxycycline or scaling and root planing on periodontal maintenance patients over 9 months. J Periodontol 2000;71(1):22-30. Scholey JM, Harrison JE. Delay and failure to publish dental research. Evid Based Dent 2005;6(3):58-61. Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med 2010;152(11):726-32. July 2015 Page 87 9. Appendices Appendix 1 – Literature searches and results The panel decided to use two strategies for the literature search to be inclusive as well as pragmatic. The first specified clinical attachment level, but did not filter by study design. The second used a filter for randomized controlled trials (RCTs), but did not specify the outcome (clinical attachment level). The purpose of the first search design was to retrieve all articles specifying clinical attachment level in the title or abstract, and the purpose of the second was to retrieve all RCTs with the keywords on periodontal disease, which could be hand-screened for applicability. Although the panel only included articles with data on clinical attachment level, they were not confident that the authors would always mention that data in the abstract. Primary Search Strategy: Hits shown for PubMed/MEDLINE Search number #1 Topic Chronic Periodontitis #2 Scaling and Root Planing #3 #6 #4 Clinical Attachment Level #1 AND #2 AND #3 Exclusions/Limits #5 #7 #8 #6 NOT #4 #7 AND #5 Syntax (((((periodontal disease [mesh]) NOT gingival diseases [mesh]) NOT peri-implantitis [mesh]) NOT periapical diseases [mesh]) NOT periodontal cyst [mesh]) OR periodontal disease [tw] OR periodontitis dental scaling [mesh] OR dental scaling [tw] OR dental prophylaxis [mesh] OR dental prophylaxis [tw] OR root planing [mesh] OR root planing [tw] OR dental cleaning OR scaling root planing OR root planing OR scaling planing OR ultrasonic therapy [mesh] OR ultrasonic therapy [tw] OR ultrasonic surgical procedures [mesh] OR ultrasonic surgical procedures [tw] OR ultrasonic scaling attachment OR periodontal atrophy [mesh] OR periodontal atrophy [tw] (animal[mh] NOT human[mh]) OR cadaver[mh] OR cadaver*[titl] OR ((comment[pt] OR editorial[pt] OR letter[pt] OR "historical article"[pt]) NOT "clinical trial"[pt]) OR addresses[pt] OR news[pt] OR "newspaper article"[pt] OR pmcbook english [lang] Hits 52861 31299 85302 1405 5336240 17861430 1325 1128 abstracts 399 RCTs Secondary Search Strategy: same as primary except CAL replaced by RCT filter: (randomized controlled trial[Publication Type] OR (randomized[Title/Abstract] AND controlled[Title/Abstract] AND trial[Title/Abstract])) with approximately 600 records found. July 2015 Page 88 Primary Search Strategy: Hits shown for Embase (10-31-12) Topic Search # #1 Syntax periodontal AND 'disease'/exp AND Hits 50,794 [humans]/lim AND [english]/lim #2 'periodontitis'/exp AND 'disease'/exp AND 18,648 [humans]/lim AND [english]/lim #3 Chronic Periodontitis 'peri implantitis'/exp AND [humans]/lim AND 136 [english]/lim #4 periapical AND 'diseases'/exp AND 4,486 [humans]/lim AND [english]/lim #5 periodontal AND 'cyst'/exp AND [humans]/lim 693 AND [english]/lim #6 #3 OR #4 OR #5 5,028 #7 #1 OR #2 50,794 #8 #7 NOT #6 #9 dental AND scaling AND [humans]/lim AND 46,747 1,854 [english]/lim #10 dental AND 'prophylaxis'/exp AND 9,799 [humans]/lim AND [english]/lim #11 root AND planing AND [humans]/lim AND 1,285 [english]/lim #12 dental AND 'cleaning'/exp AND [humans]/lim 150 AND [english]/lim #13 Scaling and Root Planing scaling AND root AND planing AND 1,099 [humans]/lim AND [english]/lim #14 scaling AND planing AND [humans]/lim AND 1,100 [english]/lim #15 'ultrasonic'/exp AND 'therapy'/exp AND 18,369 [humans]/lim AND [english]/lim #16 'ultrasonic'/exp AND surgical AND 11,979 'procedures'/exp AND [humans]/lim AND [english]/lim #17 'ultrasonic'/exp AND scaling AND 66 [humans]/lim AND [english]/lim #18 #9 OR #10 OR #11 OR #12 OR #13 OR #14 35,909 OR #15 OR #16 OR #17 Combined RCT July 2015 #19 #20 #8 AND #18 2,229 #19 AND 'randomized controlled trial'/de 526 Page 89 Secondary Search Strategy: Hits shown for Embase (10-25-12) Topic Search # #1 Syntax periodontal AND 'disease'/exp AND Hits 50,769 [humans]/lim AND [english]/lim Chronic Periodontitis #2 gingival AND 'diseases'/exp 18,203 #3 'peri implantitis'/exp 176 #4 periapical AND 'diseases'/exp 6,753 #5 periodontal AND 'cyst'/exp 1,020 #6 'periodontitis'/exp 28,928 #7 #2 OR #3 OR #4 OR #5 25,441 #8 #1 OR #6 #9 #10 #8 NOT #7 61,059 44,716 dental AND scaling AND [humans]/lim AND 1,855 [english]/lim #11 dental AND 'prophylaxis'/exp AND 9,790 [humans]/lim AND [english]/lim #12 #13 root AND planing 1,554 dental AND 'cleaning'/exp AND [humans]/lim 150 AND [english]/lim #14 Scaling and Root Planing scaling AND root AND planing AND 1,100 [humans]/lim AND [english]/lim #15 scaling AND planing 1,276 #16 'ultrasonic'/exp AND 'therapy'/exp AND 18,274 [humans]/lim AND [english]/lim #17 'ultrasonic'/exp AND surgical AND 11,920 'procedures'/exp AND [humans]/lim AND [english]/lim #18 'ultrasonic'/exp AND scaling AND 66 [humans]/lim AND [english]/lim #19 #10 OR #11 OR #12 OR #13 OR #14 OR #15 36,071 OR #16 OR #17 OR #18 #20 attachment AND [humans]/lim AND 74,675 [english]/lim #21 Clinical attachment level attachment AND level AND [humans]/lim AND 9,491 [english]/lim #22 attachment AND loss AND [humans]/lim AND 6,053 [english]/lim #23 periodontal AND 'atrophy'/exp AND 377 [humans]/lim AND [english]/lim Combined July 2015 #24 #25 #20 OR #21 OR #22 OR #23 75,040 #9 AND #19 AND #24 374 Page 90 #26 #9 AND #19 AND #24 AND [humans]/lim AND 374 [english]/lim #27 preventative AND 'dentistry'/exp AND 15 [humans]/lim AND [english]/lim #28 #1 OR #6 OR #27 61,071 #29 #30 #28 NOT #7 44,727 #19 AND #24 AND #29 374 1654 articles captured from Medline and Embase via primary and secondary searches with duplicates removed. 442 abstracts recalled for full text July 2015 Page 91 Appendix 2 – Excluded studies and reasons for exclusion Author Year Abou Sulaiman, A. E. and R. M. Shehadeh 2010 Agarwal, E., A. R. Pradeep, P. Bajaj and S. B. Naik 2012 Agarwal, E., P. Bajaj, S. B. Naik and A. R. Pradeep 2012 Ah, M. K., G. K. Johnson, W. B. Kaldahl, K. D. Patil and K. L. Kalkwarf 1994 Aimetti, M., F. Romano, I. Torta, D. Cirillo, P. Caposio and R. Romagnoli 2004 Aimetti, M., F. Romano, N. Guzzi and G. Carnevale 2011 Aitken, S., P. Birek, G. V. Kulkarni, W. L. Lee and C. A. McCulloch Akalin, F. A., E. Baltacioglu, D. Sengun, S. Hekimoglu, M. Taskin, I. Etikan and I. Fisenk Akncbay, H., S. Senel and Z. Y. Ay Alec Yen, C., P. D. Damoulis, P. C. Stark, P. L. Hibberd, M. Singh and A. S. Papas July 2015 1992 2004 2007 2008 Title Assessment of total antioxidant capacity and the use of vitamin C in the treatment of nonsmokers with chronic periodontitis Efficacy of local drug delivery of 0.5% clarithromycin gel as an adjunct to nonsurgical periodontal therapy in the treatment of current smokers with chronic periodontitis: a randomized controlled clinical trial Locally Delivered 0.5% Azithromycin, as an Adjunct to Non Surgical Treatment in Chronic Periodontitis With Type 2 Diabetes: A Randomized Controlled Clinical Trial The effect of smoking on the response to periodontal therapy Debridement and local application of tetracycline-loaded fibres in the management of persistent periodontitis: results after 12 months One-stage full-mouth disinfection as a therapeutic approach for generalized aggressive periodontitis Serial doxycycline and metronidazole in prevention of recurrent periodontitis in highrisk patients A comparative evaluation of the clinical effects of systemic and local doxycycline in the treatment of chronic periodontitis Application of chitosan gel in the treatment of chronic periodontitis The effect of a selective cyclooxygenase-2 inhibitor (celecoxib) on chronic periodontitis Journal Volume Issue Page J Periodontol 81 11 1547-54 Not 6 month study - too short J Periodontol 83 9 1155-63 Not commercially available J Periodontol Reason if Exclude Not commercially available J Clin Periodontol 21 2 91-7 Paper does not present nonsurgical data separate from all data J Clin Periodontol 31 3 166-72 Not commercially available J Periodontol 82 6 845-53 Aggressive perio J Periodontol 63 2 87-92 Study evaluated use of metronidazol for recurrent perio within 7 months of SRP plus placebo or SRP plus doxycycline. This study is really assessing the impact that placebo vs doxycycline pre tx has on subsequent metronidazol use. No comparison to SRP alone vs metronidazol. J Oral Sci 46 1 25-35 Not 6 month study - too short J Biomed Mater Res B Appl Biomater 80 2 290-6 Not commercially available J Periodontol 79 1 104-113 Duplicate citation YEN excluded anti inflammatory Page 92 Al Mubarak, S., M. Abou Rass, A. Alsuwyed, K. Al-Zoman, A. Al Sohail, S. Sobki, M. Tariq, A. Alwin Robert, S. Ciancio and P. Dandona Al-Mubarak, S., S. Ciancio, A. Aljada, P. Mohanty, C. Ross and P. Dandona Al-Katma, M. K., N. F. Bissada, J. M. Bordeaux, J. Sue and A. D. Askari 2010 A new paradigm between mechanical scaling and root planing combined with adjunctive chemotherapy for glycated hemoglobin improvement in diabetics International Journal of Diabetes Mellitus 2 3 158-164 Wrong intervention 2002 Comparative evaluation of adjunctive oral irrigation in diabetics J Clin Periodontol 29 4 295-300 Only 12 weeks in duration 2007 Control of periodontal infection reduces the severity of active rheumatoid arthritis J Clin Rheumatol 13 3 134-7 Not 6 month study - too short Alptekin, N. O., F. Kurtoglu, B. Serpek, I. Duran and M. Gozlu 2000 Effects on the clinical indices and gingival crevicular fluid enzyme activities of the cyclical regimen of low-dose doxycycline therapy for adult periodontitis J Int Acad Periodontol 2 1 8-Mar Not RCT Alves, R. V., L. Machion, M. Z. Casati, F. H. Nociti Jr, E. A. Sallum and A. W. Sallum 2005 Clinical attachment loss produced by curettes and ultrasonic scalers J Clin Periodontol 32 7 691-694 No passive control Angaji, M., S. Gelskey, G. Nogueira-Filho and D. Brothwell 2010 A systematic review of clinical efficacy of adjunctive antibiotics in the treatment of smokers with periodontitis J Periodontol 81 11 1518-28 Review Angelov, N., S. Pesevska, M. Nakova, I. Gjorgoski, K. Ivanovski, D. Angelova, O. Hoffmann and S. Andreana 2009 Periodontal treatment with a low-level diode laser: clinical findings Gen Dent 57 5 510-3 Not 6 month study - too short Apatzidou, D. A. and D. F. Kinane 2004 J Clin Periodontol 31 2 132-40 Comparison of 2 types of SRP Apatzidou, D. A., M. P. Riggio and D. F. Kinane 2004 J Clin Periodontol 31 2 141-8 No CAL data Aras, H., F. Caglayan, G. N. Guncu, A. Berberoglu and K. Kilinc 2007 J Periodontol 78 5 868-73 No CAL data Ashley, R. A. 1999 Ann N Y Acad Sci 878 335-46 Duplicate to Caton 2000 data Atieh, M. A. 2010 Lasers Med Sci 25 605-13 Review Auyeung, L., P. W. Wang, R. T. Lin, C. J. Hsieh, P. Y. Lee, R. Y. Zhuang and H. W. Chang Aykol, G., U. Baser, I. Maden, Z. Kazak, U. Onan, S. Tanrikulu- July 2015 Quadrant root planing versus same-day fullmouth root planing. I. Clinical findings Quadrant root planing versus same-day fullmouth root planing. II. Microbiological findings Effect of systemically administered naproxen sodium on clinical parameters and myeloperoxidase and elastase-like activity levels in gingival crevicular fluid Clinical trials of a matrix metalloproteinase inhibitor in human periodontal disease. SDD Clinical Research Team Photodynamic therapy as an adjunctive treatment for chronic periodontitis: a metaanalysis 4 2012 Evaluation of periodontal status and effectiveness of non-surgical treatment in patients with type 2 diabetes mellitus in Taiwan for a 1-year period J Periodontol 83 5 621-8 Not RCT. This study compared SRP among patients with diabetes. There was no placebo group that did not receive S&RP. There was no randomization because the same tx (SRP) was provided to both groups 2011 The effect of low-level laser therapy as an adjunct to non-surgical periodontal treatment J Periodontol 82 3 481-8 Experimental therapy (biostimulation) Page 93 Kucuk, E. Ademoglu, H. Issever and F. Yalcin Azarpazhooh, A., P. S. Shah, H. C. Tenenbaum and M. B. Goldberg Badersten, A., R. Nilveus and J. Egelberg Badersten, A., R. Nilveus and J. Egelberg Badersten, A., R. Nilveus and J. Egelberg 2010 1981 1984 1984 Badersten, A., R. Nilveus and J. Egelberg 1985 Bain, C. A., G. S. Beagrie, J. Bourgoin, F. Delorme, A. Holthuis, R. G. Landry, S. Roy, P. Schuller, D. Singer and R. Turnbull 1994 Bajaj, P., A. R. Pradeep, E. Agarwal, M. Kumari and S. B. Naik 2012 Beikler, T., H. Karch, B. Ehmke, B. Klaiber and T. F. Flemmig 1999 Beirne, P., A. Forgie, H. V. Worthington and J. E. Clarkson Beirne, P., H. V. Worthington and J. E. Clarkson Berglundh, T., B. Liljenberg and J. Lindhe Berkey, C. S., A. AntczakBouckoms, D. C. Hoaglin, F. Mosteller and B. L. Pihlstrom Blomlof, L., E. Bergman, A. Forsgardh, L. Foss, A. Larsson, B. Sjoberg, L. Uhlander, B. Jonsson, J. Blomlof and S. Lindskog Bogren, A., R. P. Teles, G. Torresyap, A. D. Haffajee, S. S. Socransky and J. L. Wennstrom Bogren, A., R. Teles, G. Torresyap, A. D. Haffajee, S. S. Socransky, J. Lindhe and J. L. Wennstrom July 2015 2005 2007 1999 The effect of photodynamic therapy for periodontitis: a systematic review and metaanalysis Effect of nonsurgical periodontal therapy. I. Moderately advanced periodontitis Effect of nonsurgical periodontal therapy. II. Severely advanced periodontitis Effect of nonsurgical periodontal therapy. III. Single versus repeated instrumentation Effect of nonsurgical periodontal therapy. V. Patterns of probing attachment loss in nonresponding sites The effects of spiramycin and/or scaling on advanced periodontitis in humans Locally delivered 0.5% clarithromycin, as an adjunct to nonsurgical treatment in chronic periodontitis with well-controlled type 2 diabetes: a randomized controlled clinical trial Protective effect of serum antibodies against a 110-kilodalton protein of Actinobacillus actinomycetemcomitans following periodontal therapy Routine scale and polish for periodontal health in adults Routine scale and polish for periodontal health in adults Some effects of periodontal therapy on local and systemic immunological parameters J Periodontol 81 1 14-Apr Review J Clin Periodontol 8 1 57-72 No passive control J Clin Periodontol 11 1 63-76 No passive control J Clin Periodontol 11 2 114-24 No passive control J Clin Periodontol 12 4 270-82 No passive control J Can Dent Assoc 60 3 209, 212-7 J Investig Clin Dent Oral Microbiol Immunol Spiramycin not available in USA Not commercially available 14 Cochrane Database Syst Rev Cochrane Database Syst Rev 5 281-7 1 CD004625 Review 4 CD004625 Review J Clin Periodontol 26 2 91-8 No CAL data No passive control 1995 Multiple-outcomes meta-analysis of treatments for periodontal disease J Dent Res 74 4 1030-9 Review 2000 A clinical study of root surface conditioning with an EDTA gel. I. Nonsurgical periodontal treatment Int J Periodontics Restorative Dent 20 6 560-5 24% EDTA gel. Not commercially available in US. Only 17% is available 2008 Locally delivered doxycycline during supportive periodontal therapy: a 3-year study J Periodontol 79 5 827-35 Doxycycline was provided annually after SRP 2007 A three-year prospective study of adult subjects with gingivitis. I: clinical periodontal parameters J Clin Periodontol 34 1 6-Jan No CAL data Page 94 Bonito, A. J., L. Lux and K. N. Lohr 2005 Bono, A. and M. Brunotto 2010 Borrajo, J. L., L. G. Varela, G. L. Castro, I. Rodriguez-Nunez and M. G. Torreira Bosco, J. M., B. M. Lopes, A. F. Bosco, D. M. Spolidorio and R. A. Marcantonio 2004 2009 Impact of local adjuncts to scaling and root planing in periodontal disease therapy: a systematic review Amoxicillin/metronidazole or scaling and root planing in the treatment of chronic periodontitis Diode laser (980 nm) as adjunct to scaling and root planing Local application of tetracycline solution with a microbrush: an alternative treatment for persistent periodontitis Antimicrobial irrigation of deep pockets to supplement non-surgical periodontal therapy. II. Daily irrigation Comparative clinical and microbiological effects of subgingival metronidazole application in adult periodontitis; 12-months results Clinical and microbiological effects of an essential-oil-containing mouth rinse applied in the "one-stage full-mouth disinfection" protocol--a randomized doubled-blinded preliminary study Treatment of residual pockets with photodynamic therapy, diode laser, or deep scaling. A randomized, split-mouth controlled clinical trial Evaluation of the effect of subgingival placement of chlorhexidine chips as an adjunct to scaling and root planing J Periodontol 76 8 1227-36 Review Acta Odontol Latinoam 23 3 196-203 Review Photomed Laser Surg 22 6 509-12 Not 6 month study - too short Quintessence Int 40 1 29-40 Not commercially available J Clin Periodontol 12 8 630-8 Debridement not SRP J Int Acad Periodontol 3 4 81-6 Not commercially available Clin Oral Investig 13 2 189-94 No CAL data Lasers Med Sci 27 5 979-986 Not adjuncts and no control J Periodontol 78 6 997-1001 retx with CHX chip J Clin Periodontol 28 8 782-9 Doubling Caton 2000 data Braatz, L., S. Garrett, N. Claffey and J. Egelberg 1985 Buduneli, E., A. Tunger, E. Evrenosoglu and A. Bilgic 2001 Cavalca Cortelli, S., F. Cavallini, M. F. Regueira Alves, A. Alves Bezerra, Jr., C. S. Queiroz and J. R. Cortelli 2009 Cappuyns, I., N. Cionca, P. Wick, C. Giannopoulou and A. Mombelli 2012 Carvalho, J., M. J. Novak and L. F. Mota 2007 Caton, J. G., S. G. Ciancio, T. M. Blieden, M. Bradshaw, R. J. Crout, A. F. Hefti, J. M. Massaro, A. M. Polson, J. Thomas and C. Walker 2001 Subantimicrobial dose doxycycline as an adjunct to scaling and root planing: posttreatment effects Cercek, J. F., R. D. Kiger, S. Garrett and J. Egelberg 1983 Relative effects of plaque control and instrumentation on the clinical parameters of human periodontal disease J Clin Periodontol 10 1 46-56 Not a randomized trial. There were three sequential treatments, but no control group. Chapple, I. L., A. D. Walmsley, M. S. Saxby and H. Moscrop 1992 Effect of subgingival irrigation with chlorhexidine during ultrasonic scaling J Periodontol 63 10 812-6 Compares 2 types of scalers Chapple, I. L., A. D. Walmsley, M. S. Saxby and H. Moscrop 1995 Effect of instrument power setting during ultrasonic scaling upon treatment outcome J Periodontol 66 9 756-60 No control group; measured power levels of ultrasonic scalers Chapple, I. L., M. R. Milward, N. Ling-Mountford, P. Weston, K. Carter, K. Askey, G. E. Dallal, S. 2012 Adjunctive daily supplementation with encapsulated fruit, vegetable and berry juice powder concentrates and clinical periodontal outcomes: a double-blind RCT J Clin Periodontol 39 1 62-72 Not adjunct of interest July 2015 Page 95 De Spirt, H. Sies, D. Patel and J. B. Matthews Chaves, E. S., M. K. Jeffcoat, C. C. Ryerson and B. Snyder Chee, H. K., L. P. Lim, F. Tay, A. C. Thai and C. F. Sum Chen, T., Y. Zhou, J. Liu, B. Xu, Z. Wu and D. Li Chin Quee, T., W. Al-Joburi, C. Lautar-Lemay, E. C. Chan, I. Iugovaz, J. Bourgouin and F. Delorme Christgau, M., T. Manner, S. Beuer, K. A. Hiller and G. Schmalz 2000 2008 2002 1988 2007 Christie, P., N. Claffey and S. Renvert 1998 Cionca, N., C. Giannopoulou, G. Ugolotti and A. Mombelli 2010 Claffey, N., A. Kelly, J. Bergquist and J. Egelberg 1996 Clark, D. C., S. Shenker, P. Stulginski and S. Schwartz 1983 Cobb, C. M. 2002 Cobb, C. M. 2006 Copulos, T. A., S. B. Low, C. B. Walker, Y. Y. Trebilcock and A. F. Hefti 1993 Correa, F. O., D. Goncalves, C. M. Figueredo, A. Gustafsson and S. R. Orrico 2008 Corsair, A. 1994 July 2015 Persistent bacterial colonization of Porphyromonas gingivalis, Prevotella intermedia, and Actinobacillus actinomycetemcomitans in periodontitis and its association with alveolar bone loss after 6 months of therapy Non-surgical periodontal treatment and lipid levels in diabetic patients Biological effects of hyperbaric oxygen on human severe periodontitis Comparison of spiramycin and tetracycline used adjunctively in the treatment of advanced chronic periodontitis Periodontal healing after non-surgical therapy with a new ultrasonic device: a randomized controlled clinical trial The use of 0.2% chlorhexidine in the absence of a structured mechanical regimen of oral hygiene following the non-surgical treatment of periodontitis Microbiologic testing and outcomes of fullmouth scaling and root planing with or without amoxicillin/metronidazole in chronic periodontitis Patterns of attachment loss in advanced periodontitis patients monitored following initial periodontal treatment Effectiveness of routine periodontal treatment with and without adjunctive metronidazole therapy in a sample of mentally retarded adolescents Clinical significance of non-surgical periodontal therapy: an evidence-based perspective of scaling and root planing Lasers in periodontics: a review of the literature Comparative analysis between a modified ultrasonic tip and hand instruments on clinical parameters of periodontal disease The short-term effectiveness of non-surgical treatment in reducing levels of interleukin1beta and proteases in gingival crevicular fluid from patients with type 2 diabetes mellitus and chronic periodontitis Long-term effect of tetracycline fibers on recurrent lesions in periodontal maintenance patients J Clin Periodontol 27 Ann R Australas Coll Dent Surg 19 Undersea Hyperb Med 29 J Antimicrob Chemother 12 3 22 Suppl B 897-903 No CAL data 183 Conference proceedings 159-66 Experimental treatment (hyperbaric oxygen) 171-7 No CAL data provided J Clin Periodontol 34 2 137-47 No control group J Clin Periodontol 25 1 15-23 No control group J Periodontol 81 1 15-23 No CAL data J Clin Periodontol 23 6 523-31 No control group J Periodontol 54 11 658-65 Not SRP 16-Jun Review J Clin Periodontol 29 Suppl 2 J Periodontol 77 4 545-64 Review J Periodontol 64 8 694-700 No passive control J Periodontol 79 11 2143-50 Not 6 month trial Periodontal Clin Investig 16 1 13-Aug No control Page 96 Cortelli, J. R., D. R. Aquino, S. C. Cortelli, J. Carvalho-Filho, C. V. Roman-Torres and F. O. Costa Cortelli, S. C., F. O. Costa, T. Kawai, D. R. Aquino, G. C. Franco, K. Ohara, C. V. RomanTorres and J. R. Cortelli Cortelli, S. C., J. R. Cortelli, M. Holzhausen, G. C. Franco, R. Z. Rebelo, A. S. Sonagere, S. Queiroz Cda and F. O. Costa 2008 2009 2009 Cosyn, J. and I. Wyn 2006 Cosyn, J., I. Wyn, T. De Rouck and M. M. Sabzevar 2006 Cosyn, J., I. Wyn, T. De Rouck and M. M. Sabzevar 2006 Cosyn, J., I. Wyn, T. De Rouck and M. M. Sabzevar 2007 Crespi, R., P. Cappare, I. Toscanelli, E. Gherlone and G. E. Romanos Dannewitz, B., K. Lippert, N. P. Lang, M. S. Tonetti and P. Eickholz Darby, I. B., P. J. Hodge, M. P. Riggio and D. F. Kinane Daudt, F. A., C. K. Rosing, G. A. Chiapinotto and R. V. Oppermann De Micheli, G., A. K. de Andrade, V. T. Alves, M. Seto, C. M. Pannuti and S. Cai De Micheli, G., A. K. P. De Andrade, V. T. E. Alves, M. Seto, C. M. Pannuti and S. Cai Dean, J. W., G. L. Branch-Mays, T. C. Hart, R. A. Reinhardt, B. Shapiro, E. A. Santucci and J. Lessem Del Peloso Ribeiro, E., S. Bittencourt, F. H. Nociti, Jr., E. A. Sallum, A. W. Sallum and M. Z. Casati July 2015 2007 2009 2005 2011 2011 2011 A double-blind randomized clinical trial of subgingival minocycline for chronic periodontitis Diminished treatment response of periodontally diseased patients infected with the JP2 clone of Aggregatibacter (Actinobacillus) actinomycetemcomitans Essential oils in one-stage full-mouth disinfection: double-blind, randomized clinical trial of long-term clinical, microbial and salivary effects A systematic review on the effects of the chlorhexidine chip when used as an adjunct to scaling and root planing in the treatment of chronic periodontitis Clinical benefits of subgingival chlorhexidine varnish application as an adjunct to same-day full-mouth root planing: a pilot study. Journal of Periodontology 77, 1074-1079. Long-term clinical effects of chlorhexidine varnish implemented treatment strategy for chronic periodontitis Subgingival chlorhexidine varnish administration as an adjunct to same-day fullmouth root planing. I. Clinical observations Effects of Er:YAG laser compared to ultrasonic scaler in periodontal treatment: a 2year follow-up split-mouth clinical study Supportive periodontal therapy of furcation sites: non-surgical instrumentation with or without topical doxycycline Clinical and microbiological effect of scaling and root planing in smoker and non-smoker chronic and aggressive periodontitis patients Effect of the topical application of triclosan in periodontally treated patients Efficacy of high intensity diode laser as an adjunct to non-surgical periodontal treatment: a randomized controlled trial Efficacy of high intensity diode laser as an adjunct to non-surgical periodontal treatment: A randomized controlled trial J Oral Sci 50 3 259-65 No CAL data J Clin Microbiol 47 7 2018-25 No control group J Clin Periodontol 36 4 333-42 No CAL data J Periodontol 77 2 257-64 Review J Periodontol 77 3 1074-1079. J Periodontol 77 3 406-415 J Periodontol 78 3 430-7 J Periodontol 78 7 1195-200 J Clin Periodontol 36 6 514-22 Subset of Tonetti 2012 J Clin Periodontol 32 2 200-6 No control group Acta Odontol Latinoam 24 2 205-10 Not 6 month trial Lasers Med Sci 26 1 43-8 Duplicate Lasers Med Sci 26 1 43-48 Not 6 month study - too short 2003 Topically applied minocycline microspheres: why it works Compend Contin Educ Dent 24 4 247-50, 252-7; quiz 258 2007 Comparative study of ultrasonic instrumentation for the non-surgical treatment of interproximal and non-interproximal furcation involvements J Periodontol 78 2 224-30 Not 6 month study - too short Not commercially avail in US CHX Varnish not available in US Laser not adjunct No control no CAL No control group Page 97 D'Ercole, S., R. Piccolomini, G. Capaldo, G. Catamo, G. Perinetti and L. Guida 2006 Derdilopoulou, F. V., J. Nonhoff, K. Neumann and A. M. Kielbassa 2007 Doungudomdacha, S., A. Rawlinson, T. F. Walsh and C. W. Douglas 2001 Drisko, C. H. 1998 Drisko, C. L., C. M. Cobb, W. J. Killoy, B. S. Michalowicz, B. L. Pihlstrom, R. A. Lowenguth, J. G. Caton, M. Encarnacion, M. Knowles and J. M. Goodson 1995 Dubrez, B., J. M. Graf, P. Vuagnat and G. Cimasoni 1990 Dukic, W., I. Bago, A. Aurer and M. Roguljic 2012 Eberhard, J., P. M. Jervoe-Storm, I. Needleman, H. Worthington and S. Jepsen Eberhard, J., S. Jepsen, P. M. Jervoe-Storm, I. Needleman and H. V. Worthington Ehmke, B., H. Schmidt, T. Beikler, C. Kopp, H. Karch, B. Klaiber and T. F. Flemmig Ehmke, B., A. Moter, T. Beikler, E. Milian and T. F. Flemmig Eick, S., A. Renatus, M. Heinicke, W. Pfister, S. I. Stratul and H. Jentsch Eickholz, P., T. S. Kim, T. Burklin, B. Schacher, H. H. Renggli, M. T. Schaecken, R. Holle, A. Kubler and P. Ratka-Kruger Eickholz, P., T. S. Kim, B. Schacher, P. Reitmeir, T. Burklin and P. Ratka-Kruger July 2015 Effectiveness of ultrasonic instruments in the therapy of severe periodontitis: a comparative clinical-microbiological assessment with curettes Microbiological findings after periodontal therapy using curettes, Er:YAG laser, sonic, and ultrasonic scalers Effect of non-surgical periodontal treatment on clinical parameters and the numbers of Porphyromonas gingivalis, Prevotella intermedia and Actinobacillus actinomycetemcomitans at adult periodontitis sites New Microbiol 29 2 101-10 Not randomized without proper control; compared SRP to ultrasonic J Clin Periodontol 34 7 588-98 No CAL data J Clin Periodontol 28 5 437-45 Not RCT The use of locally delivered doxycycline in the treatment of periodontitis. Clinical results J Clin Periodontol 25 11 Pt 2 947-52; discussion 978-9 Not commercially available Evaluation of periodontal treatments using controlled-release tetracycline fibers: clinical response J Periodontol 66 8 692-9 Not commercially available J Periodontol 61 12 725-31 Not RCT Increase of interproximal bone density after subgingival instrumentation: a quantitative radiographical study Clinical Effectiveness of Diode Laser Therapy as an Adjunct to Non-Surgical Periodontal Treatment: A Randomized Clinical Study J Periodontol 2008 Full-mouth treatment concepts for chronic periodontitis: a systematic review J Clin Periodontol 2008 Full-mouth disinfection for the treatment of adult chronic periodontitis Cochrane Database Syst Rev 1999 2005 2012 Clonal infection with Actinobacillus actinomycetemcomitans following periodontal therapy Adjunctive antimicrobial therapy of periodontitis: long-term effects on disease progression and oral colonization Hyaluronic Acid as an Adjunct After Scaling and Root Planing - A Prospective Randomized Clinical Trial Not 6 month trial 35 7 591-604 Review 1 CD004622 Review No CAL data J Dent Res 78 9 1518-1524 J Periodontol 76 5 749-59 J Periodontol Continuation of Flemmig 1998 trial per Philippe Not adjunct of interest 2002 Non-surgical periodontal therapy with adjunctive topical doxycycline: a double-blind randomized controlled multicenter study J Clin Periodontol 29 2 108-17 Not available commercially (concentration) 2005 Subgingival topical doxycycline versus mechanical debridement for supportive periodontal therapy: a single blind randomized controlled two-center study Am J Dent 18 6 341-6 Not adjunctive use of doxy Page 98 El-Sharkawy, H., N. Aboelsaad, M. Eliwa, M. Darweesh, M. Alshahat, A. Kantarci, H. Hasturk and T. E. Van Dyke 2010 El-Shinnawi et al. 2003 Elter, J. R., H. P. Lawrence, S. Offenbacher and J. D. Beck 1997 Engebretson, S. P., J. T. Grbic, R. Singer and I. B. Lamster 2002 Erdemir, E. O., I. Duran and S. Haliloglu 2004 Faria-Almeida, R., A. Navarro and A. Bascones 2006 Feng, H. S., C. C. Bernardo, L. L. Sonoda, F. Hayashi, G. A. Romito, L. A. De Lima, R. F. Lotufo and C. M. Pannuti 2011 Feres, M., A. D. Haffajee, K. Allard, S. Som and S. S. Socransky 2001 Feres, M., L. C. Gursky, M. Faveri, C. O. Tsuzuki and L. C. Figueiredo Flemmig, T. F., B. Epp, Z. Funkenhauser, M. G. Newman, K. S. Kornman, I. Haubitz and B. Klaiber Fourmousis, I., M. S. Tonetti, A. Mombelli, B. Lehmann, N. P. Lang and U. Bragger Friesen, L. R., K. B. Williams, L. S. Krause and W. J. Killoy 2009 The effect of alendronate sodium on alveolar bone loss in periodontitis (clinical trial) Meta-analysis of the effect of systemic metronidazole as an adjunct to scaling and root planing for adult periodontitis GCF IL-1beta profiles in periodontal disease Effects of smoking on clinical parameters and the gingival crevicular fluid levels of IL-6 and TNF-alpha in patients with chronic periodontitis Clinical and metabolic changes after conventional treatment of type 2 diabetic patients with chronic periodontitis Subgingival ultrasonic instrumentation of residual pockets irrigated with essential oils: a randomized controlled trial Change in subgingival microbial profiles in adult periodontitis subjects receiving either systemically-administered amoxicillin or metronidazole Clinical and microbiological benefits of strict supragingival plaque control as part of the active phase of periodontal therapy J Periodontol 81 11 1635-43 Not adjunct of interest J Int Acad Perio 5 1 5-10 J Periodontal Res 32 6 487-96 Review J Clin Periodontol 29 1 48-53 Not RCT J Clin Periodontol 31 2 99-104 Not RCT J Periodontol 77 4 591-8 Not RCT J Clin Periodontol 38 7 637-43 Not commercially available J Clin Periodontol 28 7 597-609 No passive control J Clin Periodontol 36 10 857-67 CHX rinse - not medical adjunct Systemic non-SDD host modulation 1995 Adjunctive supragingival irrigation with acetylsalicylic acid in periodontal supportive therapy J Clin Periodontol 22 6 427-33 No CAL data 1998 Evaluation of tetracycline fiber therapy with digital image analysis J Clin Periodontol 25 9 737-45 No CAL data J Periodontol 73 1 13-9 Not commercially available Acta Odontol Latinoam 22 3 215-9 Not 6 month trial Acta Odontol Latinoam 25 1 103-8 Not 6 month study - too short J Clin Periodontol 26 2 63-6 maintenance phase study using OTC product 2002 Funosas, E. R., L. Escovich and L. Maestri 2009 Funosas, E., G. Feser, L. Escovich and L. Maestri 2012 Furuichi, Y., B. Rosling, A. R. Volpe and J. Lindhe 1999 July 2015 Adjunctive treatment of chronic periodontitis with daily dietary supplementation with omega-3 Fatty acids and low-dose aspirin Controlled local delivery of tetracycline with polymer strips in the treatment of periodontitis The use of topical subgingival gels of nonsteroidal anti-inflammatory drugs (NSAIDs) as an adjunct to non-surgical management of chronic periodontitis Alteration of hemostasis in patients treated with subgingival NSAIDs during periodontal therapy The effect of a triclosan/copolymer dentifrice on healing after non-surgical treatment of recurrent periodontitis Page 99 Garrett, S., L. Johnson, C. H. Drisko, D. F. Adams, C. Bandt, B. Beiswanger, G. Bogle, K. Donly, W. W. Hallmon, E. B. Hancock, P. Hanes, C. E. Hawley, R. Kiger, W. Killoy, J. T. Mellonig, A. Polson, F. J. Raab, M. Ryder, N. H. Stoller, H. L. Wang, L. E. Wolinsky, G. H. Evans, C. Q. Harrold, R. M. Arnold, G. L. Southard and et al. Garrett, S., D. F. Adams, G. Bogle, K. Donly, C. H. Drisko, W. W. Hallmon, E. B. Hancock, P. Hanes, C. E. Hawley, L. Johnson, R. Kiger, W. Killoy, J. T. Mellonig, F. J. Raab, M. Ryder, N. Stoller, A. Polson, H. L. Wang, L. E. Wolinsky, R. A. Yukna, C. Q. Harrold, M. Hill, V. B. Johnson and G. L. Soouthard Garrett, S., D. F. Adams, G. Bogle, K. Donly, C. H. Drisko, W. W. Hallmon, E. B. Hancock, P. Hanes, C. E. Hawley, L. Johnson, R. Kiger, W. Killoy, J. T. Mellonig, F. J. Raab, M. Ryder, N. Stoller, A. Polson, H. L. Wang, L. E. Wolinsky, R. A. Yukna, C. Q. Harrold, M. Hill, V. B. Johnson and G. L. Southard Gebaraa, E. C., A. N. Pustiglioni, L. A. de Lima and M. P. Mayer Giannopoulou, C., E. Andersen, P. Brochut, D. Plagnat and A. Mombelli Giusti, J. S. M., C. R. Fontana, D. Gonpalves, C. Kurachi and V. Bagnato Gmur, R., U. P. Saxer and B. Guggenheim Gomes, S. C., F. B. Piccinin, C. Susin, R. V. Oppermann and R. A. Marcantonio Gomez, C., A. Dominguez, A. I. Garcia-Kass and J. A. GarciaNunez July 2015 1999 Two multi-center studies evaluating locally delivered doxycycline hyclate, placebo control, oral hygiene, and scaling and root planing in the treatment of periodontitis J Periodontol 70 5 490-503 2000 The effect of locally delivered controlledrelease doxycycline or scaling and root planing on periodontal maintenance patients over 9 months J Periodontol 71 1 22-30 Duplicate 2000 The effect of locally delivered controlledrelease doxycycline or scaling and root planing on periodontal maintenance patients over 9 months J Periodontol 71 1 22-30 Subset of Garrett 1999 Oral Health Prev Dent 1 1 29-35 No CAL data J Periodontol 77 4 707-13 No CAL data Photodiagnosis and Photodynamic Therapy 8 2 180-181 Not 6 month trial Schweiz Monatsschr Zahnmed 104 4 430-9 J Periodontol 78 8 1515-21 Not SRP Lasers Med Sci 26 4 453-63 No CAL data 2003 2006 2011 1994 2007 2011 Propolis extract as an adjuvant to periodontal treatment Enamel matrix derivative and systemic antibiotics as adjuncts to non-surgical periodontal treatment: biologic response Clinical attachment level (CAL) gain in periodontics using photodynamic therapy as adjunct treatment Effects of blunt scaling on periodontal status and subgingival microorganisms. A pilot study Effect of supragingival plaque control in smokers and never-smokers: 6-month evaluation of patients with periodontitis Adjunctive Nd:YAG laser application in chronic periodontitis: clinical, immunological, and microbiological aspects Re: Q2, doxy not used as adjunct. Re: Q1: retx at 4 months all sites regardless of status Trial not long enough between treatment and measurement 1 Page 100 Goodson, J. M., J. C. Gunsolley, S. G. Grossi, P. S. Bland, J. Otomo-Corgel, F. Doherty and J. Comiskey Gordon, J., C. Walker, C. Hovliaras and S. Socransky 2007 1990 Graziani, F., S. Cei, A. Guerrero, F. La Ferla, M. Vano, M. Tonetti and M. Gabriele 2009 Greenstein, G. 2006 Griffiths, G. S., G. J. Smart, J. S. Bulman, G. Weiss, J. Shrowder and H. N. Newman 2000 Griffiths, G. S., R. Ayob, A. Guerrero, L. Nibali, J. Suvan, D. R. Moles and M. S. Tonetti 2011 Grisi, D. C., S. L. Salvador, L. C. Figueiredo, S. L. Souza, A. B. Novaes and M. F. Grisi Grossi, S. G., J. M. Goodson, J. C. Gunsolley, J. Otomo-Corgel, P. S. Bland, F. Doherty and J. Comiskey Grossi, S. G., F. B. Skrepcinski, T. DeCaro, D. C. Robertson, A. W. Ho, R. G. Dunford and R. J. Genco 2002 Efficacy of clindamycin hydrochloride in refractory periodontitis: 24-month results Lack of short-term adjunctive effect of systemic neridronate in non-surgical periodontal therapy of advanced generalized chronic periodontitis: an open labelrandomized clinical trial Local drug delivery in the treatment of periodontal diseases: assessing the clinical significance of the results Comparison of clinical outcomes following treatment of chronic adult periodontitis with subgingival scaling or subgingival scaling plus metronidazole gel Amoxicillin and metronidazole as an adjunctive treatment in generalized aggressive periodontitis at initial therapy or retreatment: a randomized controlled clinical trial Effect of a controlled-release chlorhexidine chip on clinical and microbiological parameters of periodontal syndrome J Periodontol 78 8 1568-79 Not 6 month study - too short J Periodontol 61 11 686-91 Not an RCT J Clin Periodontol 36 5 419-27 Not commercially available in US J Periodontol 77 4 565-78 Review J Clin Periodontol 27 12 910-7 Not commercially available in US J Clin Periodontol 38 1 43-9 Aggressive perio J Clin Periodontol 29 10 875-81 Reinserted CHX chips into failing pockets at 3 and 6 months 2007 Mechanical therapy with adjunctive minocycline microspheres reduces redcomplex bacteria in smokers J Periodontol 78 9 1741-50 Not 6 month study - too short 1997 Treatment of periodontal disease in diabetics reduces glycated hemoglobin J Periodontol 68 8 713-9 Data in unusable format J Periodontol 72 3 349-53 Not 6 month study - too short J Clin Periodontol 32 3 244-53 In preference to 9 month data in Gurkan 2008 J Clin Periodontol 15 6 353-9 No control group J Clin Periodontol 35 8 696-704 Aggressive perio Gunsolley, J. C., G. M. Yeung, J. H. Butler and T. C. Waldrop 2001 Gurkan, A., S. Cinarcik and A. Huseyinov 2005 Gusberti, F. A., S. A. Syed and N. P. Lang 1988 Haas, A. N., G. D. de Castro, T. Moreno, C. Susin, J. M. Albandar, R. V. Oppermann and C. K. Rosing 2008 July 2015 Minocycline HCl microspheres reduce redcomplex bacteria in periodontal disease therapy Is loss of attachment due to root planning and scaling in sites with minimal probing depths a statistical or real occurrence? Adjunctive subantimicrobial dose doxycycline: effect on clinical parameters and gingival crevicular fluid transforming growth factor-beta levels in severe, generalized chronic periodontitis Combined antibiotic (metronidazole) and mechanical treatment effects on the subgingival bacterial flora of sites with recurrent periodontal disease Azithromycin as an adjunctive treatment of aggressive periodontitis: 12-months randomized clinical trial Page 101 Haffajee, A. D., M. A. Cugini, S. Dibart, C. Smith, R. L. Kent, Jr. and S. S. Socransky 1997 Haffajee, A. D., M. Patel and S. S. Socransky 2008 Haffajee, A. D., N. G. Uzel, E. I. Arguello, G. Torresyap, D. M. Guerrero and S. S. Socransky 2004 Haffajee, A. D., S. Dibart, R. L. Kent, Jr. and S. S. Socransky 1995 Haffajee, A. D., S. S. Socransky and J. C. Gunsolley 2003 Hallmon, W. W. and T. D. Rees 2003 Hanes, P. J. and J. P. Purvis 2003 Hanioka, T., M. Tanaka, M. Ojima, S. Shizukuishi and K. Folkers 1994 Heasman, P. A., G. I. McCracken and N. Steen 2002 Hellden, L. B., M. A. Listgarten and J. Lindhe 1979 Heller, D., V. M. Varela, M. X. Silva-Senem, M. C. Torres, E. J. Feres-Filho and A. P. Colombo 2011 Herrera, D., M. Sanz, S. Jepsen, I. Needleman and S. Roldan 2002 Hou, G. L. and C. C. Tsai 1989 July 2015 The effect of SRP on the clinical and microbiological parameters of periodontal diseases Microbiological changes associated with four different periodontal therapies for the treatment of chronic periodontitis Clinical and microbiological changes associated with the use of combined antimicrobial therapies to treat "refractory" periodontitis Clinical and microbiological changes associated with the use of 4 adjunctive systemically administered agents in the treatment of periodontal infections Systemic anti-infective periodontal therapy. A systematic review Local anti-infective therapy: mechanical and physical approaches. A systematic review Local anti-infective therapy: pharmacological agents. A systematic review Effect of topical application of coenzyme Q10 on adult periodontitis Supportive periodontal care: the effect of periodic subgingival debridement compared with supragingival prophylaxis with respect to clinical outcomes The effect of tetracycline and/or scaling on human periodontal disease Impact of systemic antimicrobials combined with anti-infective mechanical debridement on the microbiota of generalized aggressive periodontitis: a 6-month RCT A systematic review on the effect of systemic antimicrobials as an adjunct to scaling and root planing in periodontitis patients Clinical observations of the effects of nonsurgical periodontal therapy on human J Clin Periodontol 24 5 324-34 Not RCT Oral Microbiol Immunol 23 2 148-57 No CAL data J Clin Periodontol 31 10 869-77 Not RCT J Clin Periodontol 22 8 618-27 RCT comparing different systemic antibiotics and anti-inflamatory agents. BUT, a little less than half of the patients also received modified widman flap surgery. Since data is not available for those who did not have the flap surgery, cannot distinguish the effect of the surgery from the effect of the systemic agents. Ann Periodontol 8 1 115-81 Review Ann Periodontol 8 1 99-114 Review Ann Periodontol 8 1 79-98 Review Mol Aspects Med 15 Suppl s241-8 Not 6 month study - too short J Clin Periodontol 29 Suppl 3 163-72; discussion 195-6 J Clin Periodontol 6 4 222-30 Data in unusable format J Clin Periodontol 38 4 355-64 Aggressive perio J Clin Periodontol 29 Suppl 3 Gaoxiong Yi Xue Ke Xue Za Zhi 5 136-59; discussion 160-2 2 72-86 Review Review Not RCT Page 102 Hou, G. L., C. C. Tsai and A. S. Weisgold 1997 Hou, G. L., C. C. Tsai and A. S. Weisgold 1996 Hung, H. C. and C. W. Douglass 2002 Hussein, I., M. Ranka, A. Gilbert and K. Davey 2007 Ingela, L. G., A. Barbro and T. Helene 2009 Ioannou, I., N. Dimitriadis, K. Papadimitriou, D. Sakellari, I. Vouros and A. Konstantinidis 2009 Jain, R., F. Mohamed and M. Hemalatha 2012 Jansson, H., G. Bratthall and G. Soderholm 2003 Jeffcoat, M. K., K. S. Bray, S. G. Ciancio, A. R. Dentino, D. H. Fine, J. M. Gordon, J. C. Gunsolley, W. J. Killoy, R. A. Lowenguth, N. I. Magnusson, S. Offenbacher, K. G. Palcanis, H. M. Proskin, R. D. Finkelman and M. Flashner Jeffcoat, M., S. Parry, M. Sammel, B. Clothier, A. Catlin and G. Macones Jeffcoat, M. K., K. G. Palcanis, T. W. Weatherford, M. Reese, N. C. Geurs and M. Flashner Jenkins, W. M., S. H. Said, M. Radvar and D. F. Kinane Jenkins, W. M., T. W. MacFarlane, W. H. Gilmour, I. Ramsay and D. MacKenzie July 2015 1998 2011 2000 2000 1989 periodontal disease. II. Ultrasonic scaling and root planing for 6 months Ultrasonic scaling therapy in advanced periodontitis. IV. A long-term study over six years The effect of ultrasonic scaling therapy in periodontitis III. A longitudinal study over three years Meta-analysis of the effect of scaling and root planing, surgical treatment and antibiotic therapies on periodontal probing depth and attachment loss Locally delivered antimicrobials in the management of periodontitis: a critical review of the evidence for their use in practice Full-mouth versus quadrant-wise scaling clinical outcome, efficiency and treatment discomfort Hand instrumentation versus ultrasonic debridement in the treatment of chronic periodontitis: a randomized clinical and microbiological trial Minocycline containing local drug delivery system in the management of chronic periodontitis: A randomized controlled trial Clinical outcome observed in subjects with recurrent periodontal disease following local treatment with 25% metronidazole gel Adjunctive use of a subgingival controlledrelease chlorhexidine chip reduces probing depth and improves attachment level compared with scaling and root planing alone Periodontal infection and preterm birth: successful periodontal therapy reduces the risk of preterm birth Use of a biodegradable chlorhexidine chip in the treatment of adult periodontitis: clinical and radiographic findings Effect of subgingival scaling during supportive therapy Systemic metronidazole in the treatment of periodontitis Kaohsiung J Med Sci 13 2 103-16 Not RCT Kaohsiung J Med Sci 12 1 25-35 Not RCT J Clin Periodontol 29 11 975-86 Review Review Dent Update 34 8 494-496, 499496502, 505-506 Swedish dental journal 33 3 105-113 No CAL data J Clin Periodontol 36 2 132-41 No control J Indian Soc Periodontol 16 2 179-83 Not available in US - 2% mino gel J Periodontol 74 3 372-7 Not 6 month study - too short J Periodontol 69 9 989-97 CHX chip reapplied in select sites at 3 months BJOG 118 2 250-6 No CAL data J Periodontol 71 2 256-62 CHX chip reapplied in select sites at 3 months J Clin Periodontol 27 8 590-6 Not RCT J Clin Periodontol 16 7 443-50 Not an RCT Page 103 Jervoe-Storm, P. M., E. Semaan, H. AlAhdab, S. Engel, R. Fimmers and S. Jepsen 2006 Johnson, L. R., N. H. Stoller, A. Polson, C. Q. Harrold, M. Ryder and S. Garrett 2002 Jones, C. L., K. M. Milsom, P. Ratcliffe, A. Wyllie, T. V. Macfarlane and M. Tickle 2011 Jonsson, B., K. Ohrn, P. Lindberg and N. Oscarson 2010 Jordan, A. R., P. Gangler and H. P. Johren 2006 Kaldahl, W. B., K. L. Kalkwarf, K. D. Patil, M. P. Molvar and J. K. Dyer Kaldahl, W. B., K. L. Kalkwarf, K. D. Patil, M. P. Molvar and J. K. Dyer 33 3 209-15 No untreated control J Clin Periodontol 29 2 87-91 No passive control BMC Oral Health 11 J Clin Periodontol 37 10 912-9 Eur J Med Res 11 6 232-235 35 Not perio No passive control Not RCT Long-term evaluation of periodontal therapy: II. Incidence of sites breaking down J Periodontol 67 2 103-8 So SRP-only data 1996 Long-term evaluation of periodontal therapy: I. Response to 4 therapeutic modalities J Periodontol 67 2 93-102 Incomplete data Photomed Laser Surg 27 1 9-Nov Aggressive perio J Periodontol 78 7 1201-8 Aggressive perio J Clin Periodontol 34 10 880-91 No passive control Int Dent J 58 3 151-8 Not 6 month trial J Periodontol 79 11 2021-8 Review Int J Dent Hyg 5 4 225-31 No CAL data J Clin Periodontol 30 12 1024-30 OTC triclosan Clin Oral Investig 11 4 391-9 2009 Kaner, D., C. Christan, T. Dietrich, J. P. Bernimoulin, B. M. Kleber and A. Friedmann 2007 Kaner, D., J. P. Bernimoulin, W. Hopfenmuller, B. M. Kleber and A. Friedmann 2007 Kara, C., T. Demir, R. Orbak and A. Tezel 2008 Karlsson, M. R., C. I. Diogo Lofgren and H. M. Jansson 2008 Kasaj, A., A. Chiriachide and B. Willershausen 2007 July 2015 The effects of subgingival calculus on the clinical outcomes of locally-delivered controlled-release doxycycline compared to scaling and root planing Clinical outcomes of single-visit oral prophylaxis: a practice-based randomised controlled trial Evaluation of an individually tailored oral health educational programme on periodontal health Clinical treatment outcomes of periodontal therapy in HIV-seropositive patients undergoing highly active antiretroviral therapy J Clin Periodontol 1996 Kamma, J. J., V. G. Vasdekis and G. E. Romanos Kerdvongbundit, V. and U. M. Wikesjo Kim, T. S., A. Schenk, D. Lungeanu, P. Reitmeir and P. Eickholz Clinical outcomes of quadrant root planing versus full-mouth root planing 2003 2007 The effect of diode laser (980 nm) treatment on aggressive periodontitis: evaluation of microbial and clinical parameters Timing affects the clinical outcome of adjunctive systemic antibiotic therapy for generalized aggressive periodontitis Controlled-delivery chlorhexidine chip versus amoxicillin/metronidazole as adjunctive antimicrobial therapy for generalized aggressive periodontitis: a randomized controlled clinical trial Effect of Nd: YAG laser irradiation on the treatment of oral malodour associated with chronic periodontitis The effect of laser therapy as an adjunct to non-surgical periodontal treatment in subjects with chronic periodontitis: a systematic review The adjunctive use of a controlled-release chlorhexidine chip following treatment with a new ultrasonic device in supportive periodontal therapy: a prospective, controlled clinical study Effect of triclosan on healing following nonsurgical periodontal therapy in smokers Nonsurgical and surgical periodontal therapy in single-rooted teeth Not an RCT. Some patients had aggressive periodontitis Page 104 Kinane, D. F. and M. Radvar 1997 Kinane, D. F. and M. Radvar 1999 Knofler, G. U., R. E. Purschwitz and H. F. Jentsch 2007 Knofler, G. U., R. E. Purschwitz and H. F. R. Jentsch 2007 Knofler, G., R. Purschwitz, H. Jentsch, G. Birkenmeier and H. Schmidt 2008 Knofler, G. U., R. E. Purschwitz, S. Eick, W. Pfister, M. Roedel and H. F. Jentsch Kocher, T., J. Konig, P. Hansen and A. Ruhling 2011 2001 Koromantzos, P. A., K. Makrilakis, X. Dereka, S. Offenbacher, N. Katsilambros, I. A. Vrotsos and P. N. Madianos 2012 Koshy, G., Y. Kawashima, M. Kiji, H. Nitta, M. Umeda, T. Nagasawa and I. Ishikawa 2005 Kruck, C., S. Eick, G. U. Knofler, R. E. Purschwitz and H. F. Jentsch 2012 Kurtis, B., G. Tuter, M. Serdar, S. Pinar, I. Demirel and U. Toyman 2007 Kurtis, B., G. Tuter, M. Serdar, S. Pinar, I. Demirel and U. Toyman 2007 Kuru, L., B. Kuru, U. Noyan, A. Kukrer, T. Acar and S. Yilmaz 2012 L, M. S., D. C. Andia, M. Z. Casati, F. H. Nociti, Jr., E. A. Sallum, J. Gollwitzer and C. B. Walker 2007 July 2015 The effect of smoking on mechanical and antimicrobial periodontal therapy A six-month comparison of three periodontal local antimicrobial therapies in persistent periodontal pockets Clinical evaluation of partial- and full-mouth scaling in the treatment of chronic periodontitis Clinical evaluation of partial- and full-mouth scaling in the treatment of chronic periodontitis Gingival crevicular fluid levels of aspartate aminotransferase and alpha2-macroglobulin before and after topical application of metronidazole or scaling and root planing Microbiologic findings 1 year after partial- and full-mouth scaling in the treatment of moderate chronic periodontitis Subgingival polishing compared to scaling with steel curettes: a clinical pilot study Effect of non-surgical periodontal therapy on C-reactive protein, oxidative stress, and matrix metalloproteinase (MMP)-9 and MMP-2 levels in patients with type 2 diabetes: a randomized controlled study Effects of single-visit full-mouth ultrasonic debridement versus quadrant-wise ultrasonic debridement Clinical and microbiologic results 12 months after scaling and root planing with different irrigation solutions in patients with moderate chronic periodontitis: a pilot randomized trial GCF MMP-8 levels in smokers and nonsmokers with chronic periodontitis following scaling and root planing accompanied by systemic use of flurbiprofen Gingival crevicular fluid prostaglandin E(2) and thiobarbituric acid reactive substance levels in smokers and non-smokers with chronic periodontitis following phase I periodontal therapy and adjunctive use of flurbiprofen Effects of adjunctive local or systemic metronidazole with non-surgical periodontal therapy on periodontal clinical parameters and gingival crevicular fluid biomarkers Microbiologic changes following administration of locally delivered doxycycline in smokers: a 15-month follow-up J Periodontol 68 5 467-72 Not 6 month study - too short J Periodontol 70 1 7-Jan Tx not commercially avaiable J Periodontol 78 11 2135-42 J Periodontol 78 11 2135-2142 Comparing SRP methods Quintessence Int 39 5 381-9 Elyzol not available in US Quintessence Int 42 9 e107-17 J Clin Periodontol 28 2 194-9 Not an RCT J Periodontol 83 1 10-Mar Data in unusable format (% reduction from baseline) J Clin Periodontol 32 7 734-43 No passive control J Periodontol 83 3 312-20 Duplicate J Periodontol 78 10 1954-61 Not 6 month study - too short J Periodontol 78 1 104-11 Not 6 month study - too short Nobel Medicus 8 1 89-94 Not 6 month study - too short J Periodontol 78 11 2143-9 No new data (Machion 2004, 2006) Duplicate CHX gel not available in US Page 105 Lane, N., G. C. Armitage, P. Loomer, S. Hsieh, S. Majumdar, H. Y. Wang, M. Jeffcoat and T. Munoz 2005 Lang, N. P., W. C. Tan, M. A. Krahenmann and M. Zwahlen 2008 Lee, J. Y., Y. M. Lee, S. Y. Shin, Y. J. Seol, Y. Ku, I. C. Rhyu, C. P. Chung and S. B. Han 2004 Leiknes, T., K. N. Leknes, O. E. Boe, R. J. Skavland and T. Lie 2007 Lekovic, V., E. B. Kenney, F. A. Carranza, Jr. and B. Endres 1983 Leonhardt, A., C. Bergstrom, L. Krok and G. Cardaropoli 2006 Leonhardt, A., C. Bergstrom, L. Krok and G. Cardaropoli 2007 Leyes Borrajo, J. L., L. Garcia Varela, G. Lopez Castro, I. Rodriguez-Nunez and M. G. Torreira 2004 Lie, T., G. Bruun and O. E. Boe 1998 Lin, S. J., Y. K. Tu, S. C. Tsai, S. M. Lai and H. K. Lu 2012 Loesche, W. J., S. A. Syed, E. C. Morrison, G. A. Kerry, T. Higgins and J. Stoll Loesche, W. J., E. Schmidt, B. A. Smith, E. C. Morrison, R. Caffesse and P. P. Hujoel Loggner Graff, I., B. Asklow and H. Thorstensson Loos, B., R. Kiger and J. Egelberg Lopez, N. J., A. Quintero, P. A. Casanova, C. I. Ibieta, V. Baelum and R. Lopez July 2015 Bisphosphonate therapy improves the outcome of conventional periodontal treatment: results of a 12-month, randomized, placebo-controlled study A systematic review of the effects of fullmouth debridement with and without antiseptics in patients with chronic periodontitis Effect of subantimicrobial dose doxycycline as an effective adjunct to scaling and root planing Topical use of a metronidazole gel in the treatment of sites with symptoms of recurring chronic inflammation The effect of metronidazole on human periodontal disease. A clinical and bacteriological study Healing following ultrasonic debridement and PVP-iodine in individuals with severe chronic periodontal disease: a randomized, controlled clinical study Microbiological effect of the use of an ultrasonic device and iodine irrigation in patients with severe chronic periodontal disease: a randomized controlled clinical study Diode laser (980 nm) as adjunct to scaling and root planing Effects of topical metronidazole and tetracycline in treatment of adult periodontitis Non-surgical periodontal therapy with and without subgingival minocycline administration in patients with poorly controlled type II diabetes: a randomized controlled clinical trial J Periodontol 76 7 1113-22 Experimental therapy J Clin Periodontol 35 8 Suppl 21-Aug Review J Periodontol 75 11 1500-8 Adjunct more than 1 week after SRP J Periodontol 78 8 1538-44 Not available US J Periodontol 54 8 476-80 Not 6 month study - too short Acta Odontol Scand 64 5 262-6 Debridement not SRP Acta Odontol Scand 65 1 52-9 No CAL data Photomed Laser Surg 22 6 509-512 Not 6 month trial J Periodontol 69 7 819-27 Not commercially available Clin Oral Investig 16 2 599-609 Not commercially available in US 1984 Metronidazole in periodontitis. I. Clinical and bacteriological results after 15 to 30 weeks J Periodontol 55 6 325-35 CCT 1991 Effects of metronidazole on periodontal treatment needs J Periodontol 62 4 247-57 CAL data not at 6 months Swed Dent J 33 3 105-13 Full vs quadrant no control J Clin Periodontol 14 1 29-33 No passive control J Periodontol 83 3 267-78 Duplicate 2009 1987 2012 Full-mouth versus quadrant-wise scaling-clinical outcome, efficiency and treatment discomfort An evaluation of basic periodontal therapy using sonic and ultrasonic scalers Effects of periodontal therapy on systemic markers of inflammation in patients with metabolic syndrome: a controlled clinical trial Page 106 Lopez, N. J., A. Quintero, P. A. Casanova, C. I. Ibieta, V. Baelum and R. Lopezi 2012 Lulic, M., I. Leiggener Gorog, G. E. Salvi, C. A. Ramseier, N. Mattheos and N. P. Lang 2009 Lundstrom, A., L. A. Johansson and S. E. Hamp 1984 MacAlpine, R., I. Magnusson, R. Kiger, M. Crigger, S. Garrett and J. Egelberg Machtei, E. E., E. Hausmann, M. Schmidt, S. G. Grossi, R. Dunford, R. Schifferle, K. Munoz, G. Davies, J. Chandler and R. J. Genco 1985 Effects of periodontal therapy on systemic markers of inflammation in patients with metabolic syndrome: A controlled clinical trial One-year outcomes of repeated adjunctive photodynamic therapy during periodontal maintenance: a proof-of-principle randomizedcontrolled clinical trial Effect of combined systemic antimicrobial therapy and mechanical plaque control in patients with recurrent periodontal disease Antimicrobial irrigation of deep pockets to supplement oral hygiene instruction and root debridement. I. Bi-weekly irrigation J Periodontol 83 3 267-278 No SRP control group J Clin Periodontol 36 8 661-6 Maintenance study with mixed tx J Clin Periodontol 11 5 321-30 No CAL data J Clin Periodontol 12 7 568-77 Debridement not SRP Not RCT 1998 Radiographic and clinical responses to periodontal therapy J Periodontol 69 5 590-5 Machtei, E. E., I. Hirsh, M. Falah, E. Shoshani, A. Avramoff and A. Penhasi 2011 Multiple applications of flurbiprofen and chlorhexidine chips in patients with chronic periodontitis: a randomized, double blind, parallel, 2-arms clinical trial J Clin Periodontol 38 11 1037-43 Head to head- no control Machtei, E. E., M. Schmidt, E. Hausmann, S. Grossi, R. Dunford, G. Davies, J. Chandler and R. J. Genco 2000 Outcome variables in periodontal research: means and threshold-based site changes J Periodontol 71 4 555-61 Not RCT Madden, T. E., B. Herriges, L. D. Boyd, G. Laughlin, G. Chiodo and D. Rosenstein 2008 J Contemp Dent Pract 9 5 16-Sep Not properly controlled Magnusson, I., W. B. Clark, S. B. Low, J. Maruniak, R. G. Marks and C. B. Walker 1989 J Clin Periodontol 16 10 647-53 Not RCT J Clin Periodontol 21 9 628-37 Not randomized allocation to antibiotics; Repeated SRP in all pts Photomed Laser Surg 30 3 160-6 No CAL data J Periodontal Res 41 6 503-12 Not RCT J Clin Periodontol 35 10 885-896 Not 6 month trial Magnusson, I., S. B. Low, W. P. McArthur, R. G. Marks, C. B. Walker, J. Maruniak, M. Taylor, P. Padgett, J. Jung and W. B. Clark Makhlouf, M., M. M. Dahaba, J. Tuner, S. A. Eissa and T. A. Harhash 1994 2012 Mantyla, P., M. Stenman, D. Kinane, T. Salo, K. Suomalainen, S. Tikanoja and T. Sorsa 2006 Matarazzo, F., L. C. Figueiredo, S. E. B. Cruz, M. Faveri and M. Feres 2008 July 2015 Alterations in HbA1c following minimal or enhanced non-surgical, non-antibiotic treatment of gingivitis or mild periodontitis in type 2 diabetic patients: a pilot trial Effect of non-surgical periodontal therapy combined with adjunctive antibiotics in subjects with "refractory" periodontal disease. (I). Clinical results Treatment of subjects with refractory periodontal disease Effect of adjunctive low level laser therapy (LLLT) on nonsurgical treatment of chronic periodontitis Monitoring periodontal disease status in smokers and non-smokers using a gingival crevicular fluid matrix metalloproteinase-8specific chair-side test Clinical and microbiological benefits of systemic metronidazole and amoxicillin in the treatment of smokers with chronic periodontitis: A randomized placebo-controlled study Page 107 Matarazzo, F., L. C. Figueiredo, S. E. Cruz, M. Faveri and M. Feres 2008 Matesanz, P., D. Herrera, A. Echeverria, A. O'Connor, I. Gonzalez and M. Sanz 2012 Matisko, M. W. and N. F. Bissada 1993 Matsumoto, S., H. Ogawa, S. Soda, S. Hirayama, N. Amarasena, Y. Aizawa and H. Miyazaki Maze, G. I., R. A. Reinhardt, R. K. Agarwal, J. K. Dyer, D. H. Robinson, L. M. DuBois, G. J. Tussing and C. R. Maze McColl, E., K. Patel, G. Dahlen, M. Tonetti, F. Graziani, J. Suvan and L. Laurell Mdala, I., A. D. Haffajee, S. S. Socransky, B. F. de Blasio, M. Thoresen, I. Olsen and J. M. Goodson Mendonca, A. C., V. R. Santos, F. V. Ribeiro, J. A. Lima, T. S. Miranda, M. Feres and P. M. Duarte Mestnik, M. J., M. Feres, L. C. Figueiredo, G. Soares, R. P. Teles, D. Fermiano, P. M. Duarte and M. Faveri Michalowicz, B. S., B. L. Pihlstrom, C. L. Drisko, C. M. Cobb, W. J. Killoy, J. G. Caton, R. A. Lowenguth, C. Quinones, M. Encarnacion, M. Knowles and et al. Michalowicz, B. S., B. L. Pihlstrom, C. L. Drisko, C. M. Cobb, W. J. Killoy, J. G. Caton, R. A. Lowenguth, C. Quinones, M. Encarnacion, M. Knowles and J. M. Goodson July 2015 Clinical and microbiological benefits of systemic metronidazole and amoxicillin in the treatment of smokers with chronic periodontitis: a randomized placebo-controlled study A randomized clinical trial on the clinical and microbiological efficacy of a xanthan gel with chlorhexidine for subgingival use Short-term sequential administration of amoxicillin/clavulanate potassium and doxycycline in the treatment of recurrent/progressive periodontitis J Clin Periodontol 35 10 885-96 Clin Oral Investig Duplicate Not available in US J Periodontol 64 6 553-8 Incorrect control group 2009 Effect of antimicrobial periodontal treatment and maintenance on serum adiponectin in type 2 diabetes mellitus J Clin Periodontol 36 2 142-8 No CAL data 1995 Response to intracrevicular controlled delivery of 25% tetracycline from poly(lactide/glycolide) film strips in SPT patients J Clin Periodontol 22 11 860-7 Tetracycline film strips not commercially available J Clin Periodontol 33 2 141-50 No passive control J Oral Microbiol 4 J Clin Periodontol 39 4 368-76 No passive control J Clin Periodontol 39 10 955-61 Aggressive perio 2006 2012 2012 2012 Supportive periodontal therapy using mechanical instrumentation or 2% minocycline gel: a 12 month randomized, controlled, single masked pilot study Multilevel analysis of clinical parameters in chronic periodontitis after root planing/scaling, surgery, and systemic and local antibiotics: 2year results Surgical and non-surgical therapy with systemic antimicrobials for residual pockets in type 2 diabetics with chronic periodontitis: a pilot study The effects of adjunctive metronidazole plus amoxicillin in the treatment of generalized aggressive periodontitis: a 1-year doubleblinded, placebo-controlled, randomized clinical trial No new data (Goodson 2012) 1995 Evaluation of periodontal treatments using controlled-release tetracycline fibers: maintenance response J Periodontol 66 8 708-15 Duplicate 1995 Evaluation of periodontal treatments using controlled-release tetracycline fibers: Maintenance response J Periodontol 66 8 708-715 Not commercially available Page 108 Mizrak, T., G. N. Guncu, F. Caglayan, T. A. Balci, G. S. Aktar and F. Ipek 2006 Mombelli, A., B. Lehmann, M. Tonetti and N. P. Lang 1997 Mombelli, A., F. A. Gusberti and N. P. Lang 1989 Mongardini, C., D. van Steenberghe, C. Dekeyser and M. Quirynen 1999 Moreira, R. M. and E. J. FeresFilho 2007 Muller, H. P., J. Hartmann and L. Flores-de-Jacoby 1986 Nassar, P. O., C. S. Walker, C. S. Salvador, F. A. Felipetti, S. R. Orrico and C. A. Nassar 2012 Nassrawin, N. A. 2010 Newman, M. G., K. S. Kornman and F. M. Doherty 1994 Nogueira-Filho, G. R., B. T. Rosa and J. R. David-Neto Novak, M. J., D. R. Dawson, 3rd, I. Magnusson, K. Karpinia, A. Polson, A. Polson, M. E. Ryan, S. Ciancio, C. H. Drisko, D. Kinane, C. Powala and M. Bradshaw Obeid, P. R., W. D'Hoore and P. Bercy Offenbacher, S., J. D. Beck, K. Moss, L. Mendoza, D. W. Paquette, D. A. Barrow, D. J. Couper, D. D. Stewart, K. L. Falkner, S. P. Graham, S. Grossi, J. C. Gunsolley, T. Madden, G. Maupome, M. Trevisan, T. E. Van Dyke and R. J. Genco July 2015 Effect of a controlled-release chlorhexidine chip on clinical and microbiological parameters and prostaglandin E2 levels in gingival crevicular fluid Clinical response to local delivery of tetracycline in relation to overall and local periodontal conditions Treatment of recurrent periodontal disease by root planing and Ornidazole (Tiberal). Clinical and microbiological findings One stage full- versus partial-mouth disinfection in the treatment of chronic adult or generalized early-onset periodontitis. I. Longterm clinical observations Comparison between full-mouth scaling and root planing and quadrant-wise basic therapy of aggressive periodontitis: 6-month clinical results Clinical alterations in relation to the morphological composition of the subgingival microflora following scaling and root planing J Periodontol 77 3 437-43 Retreat with chip at 3 months J Clin Periodontol 24 7 470-7 Not commercially available J Clin Periodontol 16 1 38-45 No control group J Periodontol 70 6 632-45 CHX gel not available in US J Periodontol 78 9 1683-8 Aggressive perio J Clin Periodontol 13 9 825-32 Not randomized Diabetes Res Clin Pract 96 1 35-9 No passive control Eastern Mediterranean Health Journal 16 2 162-165 No passive control J Periodontol 65 7 685-91 Not commercially available Undersea Hyperb Med 37 2 107-14 Experimental treatment (hyperbaric oxygen) 2008 Combining host modulation and topical antimicrobial therapy in the management of moderate to severe periodontitis: a randomized multicenter trial J Periodontol 79 1 33-41 Experimental therapy (localsystemic combined) 2004 Comparative clinical responses related to the use of various periodontal instrumentation J Clin Periodontol 31 3 193-9 No passive control 2010 2009 Lipid profile of people with diabetes mellitus type 2 and periodontal disease Effect of smoking on the response to nonsurgical periodontal therapy A 6-month multi-center evaluation of adjunctive tetracycline fiber therapy used in conjunction with scaling and root planing in maintenance patients: clinical results Effects of hyperbaric oxygen therapy on the treatment of severe cases of periodontitis Results from the Periodontitis and Vascular Events (PAVE) Study: a pilot multicentered, randomized, controlled trial to study effects of periodontal therapy in a secondary prevention model of cardiovascular disease J Periodontol 80 2 190-201 No appriate control group. This study compared SRP to a "community group" that was provided no treatment. But community group was given a referral for perio treatment to be provided elsewhere, and some in that group received prophy, SRP and/or perio surgery. Page 109 Oliveira (de Lima Oliviera), A. P., M. de Faveri, L. C. Gursky, M. J. Mestnik, M. Feres, A. D. Haffajee, S. S. Socransky and R. P. Teles Oringer, R. J., K. F. Al-Shammari, W. A. Aldredge, V. J. Iacono, R. M. Eber, H. L. Wang, B. Berwald, R. Nejat and W. V. Giannobile Oringer, R. J., T. E. Van Dyke and J. Lessem Oz, S. G., O. Fentoglu, A. Kilicarslan, G. S. Guven, M. D. Tanrtover, Y. Aykac and T. Sozen Paolantonio, M., S. D'Ercole, A. Pilloni, D. D'Archivio, L. Lisanti, F. Graziani, B. Femminella, G. Sammartino, L. Perillo, S. Tete, G. Perfetti, G. Spoto, R. Piccolomini and G. Perinetti Papapanou, P. N., A. M. Neiderud, E. Disick, E. Lalla, G. C. Miller and G. Dahlen 2012 Effects of periodontal therapy on GCF cytokines in generalized aggressive periodontitis subjects J Clin Periodontol 39 3 295-302 Aggressive perio 2002 Effect of locally delivered minocycline microspheres on markers of bone resorption J Periodontol 73 8 835-42 No CAL data 2002 The challenge of treating periodontal patients who smoke--the efficacy of Arestin J Int Acad Periodontol 4 3 89-94 Subpopulation of 2 RCTs mixed 2007 Beneficial effects of periodontal treatment on metabolic control of hypercholesterolemia South Med J 100 7 686-91 3-month study 2009 Clinical, microbiologic, and biochemical effects of subgingival administration of a Xanthan-based chlorhexidine gel in the treatment of periodontitis: a randomized multicenter trial J Periodontol 80 9 1479-92 CHX gel not available in US 2004 Longitudinal stability of serum immunoglobulin G responses to periodontal bacteria J Clin Periodontol 31 11 985-90 No control group Papli, R. and J. M. Lewis 1989 Refractory chronic periodontitis: effect of oral tetracycline hydrochloride and root planning Aust Dent J 34 1 60-8 Paquette, D., R. Oringer, J. Lessem, S. Offenbacher, R. Genco, G. R. Persson, E. A. Santucci and R. C. Williams 2003 Locally delivered minocycline microspheres for the treatment of periodontitis in smokers J Clin Periodontol 30 9 787-94 No CAL data Perinetti, G., M. Paolantonio, C. Cordella, S. D'Ercole, E. Serra and R. Piccolomini 2004 J Clin Periodontol 31 4 273-81 CHX gel not avail US J Periodontol 74 6 916-932 Review J Periodontol 75 6 830-838 Review J Periodontol 74 6 916-32 Duplicate J Periodontol 75 6 830-8 Duplicate J Clin Periodontol 21 2 107-12 Debridement not SRP J Clin Periodontol 19 9 Pt 2 715-22 Not available in US J Clin Periodontol 25 4 322-9 Prevention study Pavia, M., C. G. A. Nobile and I. F. Angelillo Pavia, M., C. G. A. Nobile, A. Bianco and I. F. Angelillo Pavia, M., C. G. Nobile and I. F. Angelillo Pavia, M., C. G. Nobile, A. Bianco and I. F. Angelillo 2003 2004 2003 2004 Pavicic, M. J., A. J. van Winkelhoff, N. H. Douque, R. W. Steures and J. de Graaff 1994 Pedrazzoli, V., M. Kilian and T. Karring 1992 Persson, R. E., G. R. Persson, L. V. Powell and H. A. Kiyak 1998 July 2015 Clinical and microbiological effects of subgingival administration of two active gels on persistent pockets of chronic periodontitis patients Meta-analysis of local tetracycline in treating chronic periodontitis Meta-analysis of local metronidazole in the treatment of chronic periodontitis Meta-analysis of local tetracycline in treating chronic periodontitis Meta-analysis of local metronidazole in the treatment of chronic periodontitis Microbiological and clinical effects of metronidazole and amoxicillin in Actinobacillus actinomycetemcomitans-associated periodontitis. A 2-year evaluation Comparative clinical and microbiological effects of topical subgingival application of metronidazole 25% dental gel and scaling in the treatment of adult periodontitis Periodontal effects of a biobehavioral prevention program Not RCT Page 110 1983 Comparison of surgical and nonsurgical treatment of periodontal disease. A review of current studies and additional results after 61/2 years J Clin Periodontol 10 5 524-41 Review Pihlstrom, B. L., L. F. Wolff, M. B. Bakdash, E. M. Schaffer, J. R. Jensen, Jr., D. M. Aeppli and C. L. Bandt 1987 Salt and peroxide compared with conventional oral hygiene. I. Clinical results J Periodontol 58 5 291-300 SRP not performed Pons-Vicente, O., E. ValmasedaCastellon, L. Berini-Aytes and C. Gay-Escoda 2009 Oral Surg Oral Med Oral Pathol Oral Radiol Endod 107 3 e11-9 Not chronic perio Pradeep, A. R. and M. S. Thorat 2010 J Periodontol 81 2 214-22 Not adjunct of interest Pihlstrom, B. L., R. B. McHugh, T. H. Oliphant and C. Ortiz-Campos Pradeep, A. R., M. Kumari, N. S. Rao, S. S. Martande and S. B. Naik Pradeep, A. R., N. Kalra, N. Priyanka and S. B. Naik 2012 2012 Pradeep, A. R., N. S. Rao, P. Bajaj and M. Kumari 2012 Pradeep, A. R., N. S. Rao and S. B. Naik 2012 Pradeep, A. R., A. Sharma, N. S. Rao, P. Bajaj, S. B. Naik and M. Kumari 2012 Pradeep, A. R., N. Kalra, N. Priyanka, E. Khaneja, S. B. Naik and S. P. Singh 2012 Preshaw, P. M., A. F. Hefti and M. H. Bradshaw 2005 Preshaw, P. M., M. J. Novak, J. Mellonig, I. Magnusson, A. Polson, W. V. Giannobile, R. W. Rowland, J. Thomas, C. Walker, D. R. Dawson, D. Sharkey and M. H. Bradshaw 2008 Purucker, P., H. Mertes, J. M. Goodson and J. P. Bernimoulin 2001 July 2015 Effect on pocket depth and attachment level of manual versus ultrasonic scaling of lower second molars following lower third molar extraction: a randomized controlled trial Clinical effect of subgingivally delivered simvastatin in the treatment of patients with chronic periodontitis: a randomized clinical trial Clinical Efficacy of Subgingivally Delivered 1.2% Atorvastatin in Chronic Periodontitis: A Randomized Controlled Clinical Trial Microbiological outcomes of systemic ornidazole use in chronic periodontitis. Part II Efficacy of Subgingivally Delivered Simvastatin in the Treatment of Type 2 Diabetes Subjects With Chronic Periodontitis: A Randomized Double Blinded Controlled Clinical Trial Efficacy of Varying Concentrations of Subgingivally Delivered Metformin in the Treatment of Chronic Periodontitis: A Randomized Controlled Clinical Trial Local drug delivery of alendronate gel for the treatment of patients with chronic periodontitis with diabetes mellitus: a double-masked controlled clinical trial Systemic ornidazole as an adjunct to nonsurgical periodontal therapy in the treatment of chronic periodontitis: a randomized, doublemasked, placebo-controlled clinical trial Adjunctive subantimicrobial dose doxycycline in smokers and non-smokers with chronic periodontitis J Periodontol J Int Acad Periodontol Not adjunct of interest 14 2 50-4 No CAL data J Periodontol Not adjunct of interest J Periodontol Not available commercially J Periodontol 83 10 1322-8 Not available commercially J Periodontol 83 9 1149-54 Retx all pts at all intervals and not just failing sites J Clin Periodontol 32 6 610-6 Retrospective analysis Modified-release subantimicrobial dose doxycycline enhances scaling and root planing in subjects with periodontal disease J Periodontol 79 3 440-52 Systemic sustained release SDD Doxy - experimental Local versus systemic adjunctive antibiotic therapy in 28 patients with generalized aggressive periodontitis J Periodontol 72 9 1241-5 Aggressive perio Page 111 Qadri, T., F. Javed, P. Poddani, J. Tuner and A. Gustafsson Quee, T. C., E. C. Chan, C. Clark, C. Lautar-Lemay, M. J. Bergeron, J. Bourgouin and J. Stamm Quirynen, M., C. Soers, M. Desnyder, C. Dekeyser, M. Pauwels and D. van Steenberghe Quirynen, M., C. Mongardini, M. de Soete, M. Pauwels, W. Coucke, J. van Eldere and D. van Steenberghe Quirynen, M., M. De Soete, G. Boschmans, M. Pauwels, W. Coucke, W. Teughels and D. van Steenberghe Ramfjord, S. P., R. G. Caffesse, E. C. Morrison, R. W. Hill, G. J. Kerry, E. A. Appleberry, R. R. Nissle and D. L. Stults Rassameemasmaung, S., A. Sirikulsathean, C. Amornchat, P. Maungmingsook, P. Rojanapanthu and W. Gritsanaphan Ratka-Kruger, P., D. Mahl, D. Deimling, J. S. Monting, I. Jachmann, E. Al-Machot, A. Sculean, M. Berakdar, P. M. Jervoe-Storm and A. Braun Reddy, M. S., K. G. Palcanis, M. L. Barnett, S. Haigh, C. H. Charles and M. K. Jeffcoat 2011 1987 2005 2000 Lasers Med Sci 26 6 763-6 No CAL data J Periodontol 58 9 594-601 Not available in USA J Clin Periodontol 32 4 390-400 No CAL data J Clin Periodontol 27 8 578-89 CHX gel not available in US 2006 Benefit of "one-stage full-mouth disinfection" is explained by disinfection and root planing within 24 hours: a randomized controlled trial J Clin Periodontol 33 9 639-47 Not available in US (Corsodyl) 1987 4 modalities of periodontal treatment compared over 5 years J Clin Periodontol 14 8 445-52 No passive control 2008 Topical application of Garcinia mangostana L. pericarp gel as an adjunct to periodontal treatment Complement Ther Med 16 5 262-7 Not 6 month trial 2012 Er:YAG laser treatment in supportive periodontal therapy J Clin Periodontol 39 5 483-9 No passive control J Clin Periodontol 20 9 635-40 Anti-inflammatory Ann Periodontol 8 1 Dec-37 Review J Periodontol 62 5 317-21 Not adjunctive J Periodontol 67 6 562-71 No passive control J Periodontol 68 4 346-52 Not randomized 1993 Reddy, M. S., N. C. Geurs and J. C. Gunsolley 2003 Reinhardt, R. A., G. K. Johnson and L. M. DuBois 1991 Renvert, S., G. Dahlen and M. Wikstrom 1996 Renvert, S., G. Dahlen and B. Snyder 1997 July 2015 Long-term effects of a single application of a water-cooled pulsed Nd:YAG laser in supplement to scaling and root planing in patients with periodontal inflammation The role of adjunctive Rodogyl therapy in the treatment of advanced periodontal disease. A longitudinal clinical and microbiologic study A 0.05% cetyl pyridinium chloride/0.05% chlorhexidine mouth rinse during maintenance phase after initial periodontal therapy The role of chlorhexidine in the one-stage fullmouth disinfection treatment of patients with advanced adult periodontitis. Long-term clinical and microbiological observations Efficacy of meclofenamate sodium (Meclomen) in the treatment of rapidly progressive periodontitis Periodontal host modulation with antiproteinase, anti-inflammatory, and bonesparing agents. A systematic review Clinical effects of closed root planing compared to papilla reflection and fiber optic augmentation Treatment of periodontal disease based on microbiological diagnosis. Relation between microbiological and clinical parameters during 5 years Clinical and microbiological effects of subgingival antimicrobial irrigation with citric acid as evaluated by an enzyme immunoassay and culture analysis Page 112 Ribeiro (Del Peloso Ribeiro), E., S. Bittencourt, G. M. Ambrosano, F. H. Nociti, Jr., E. A. Sallum, A. W. Sallum and M. Z. Casati Ribeiro Edel, P., S. Bittencourt, E. A. Sallum, A. W. Sallum, F. H. Nociti, Jr. and M. Z. Casati Rodrigues, I. F., L. Machion, M. Z. Casati, F. H. Nociti, Jr., S. de Toledo, A. W. Sallum and E. A. Sallum 2006 Povidone-iodine used as an adjunct to nonsurgical treatment of furcation involvements J Periodontol 77 2 211-7 Retreated failing sites as per initial therapy 2010 Non-surgical instrumentation associated with povidone-iodine in the treatment of interproximal furcation involvements J Appl Oral Sci 18 6 599-606 Retreated failing sites as per initial therapy 2007 Clinical evaluation of the use of locally delivered chlorhexidine in periodontal maintenance therapy J Periodontol 78 4 624-8 Chip placed 3 mo. After baseline so not adjunct; second chip not adjunct but tx alone Minerva Stomatol 54 2-Jan 43-51 Not commercially available Lasers Med Sci 25 6 891-9 Not 6 month trial J Clin Periodontol 29 4 342-50 Unusable data format Romano, F., I. Torta, C. Debernardi and M. Aimetti 2005 Romeo, U., G. Palaia, R. Botti, V. Leone, J. P. Rocca and A. Polimeni 2010 Rooney, J., W. G. Wade, S. V. Sprague, R. G. Newcombe and M. Addy 2002 Rosa, E. F., P. Corraini, V. F. De Carvalho, G. Inoue, E. F. Gomes, J. P. B. Lotufo, G. De Micheli and C. M. Pannuti Rosa, E. F., P. Corraini, V. F. de Carvalho, G. Inoue, E. F. Gomes, J. P. Lotufo, G. De Micheli and C. M. Pannuti Rosling, B. G., J. Slots and R. L. Webber Rosling, B. G., J. Slots, R. L. Webber, L. A. Christersson and R. J. Genco Rosling, B., M. K. Hellstrom, P. Ramberg, S. S. Socransky and J. Lindhe Rudhart, A., P. Purucker, A. Kage, W. Hopfenmuller and J. P. Bernimoulin Rupf, S., I. Brader, D. Vonderlind, S. Kannengiesser, K. Eschrich, I. Roeder and K. Merte Ryder, M. I., B. Pons, D. Adams, B. Beiswanger, V. Blanco, G. Bogle, K. Donly, W. Hallmon, E. B. Hancock, P. Hanes, C. Hawley, L. Johnson, H. L. Wang, July 2015 Debridement and local application of tetracycline in the management of persistent periodontitis. Clinical and microbiological results after 12 months Non-surgical periodontal therapy assisted by potassium-titanyl-phosphate laser: a pilot study Adjunctive effects to non-surgical periodontal therapy of systemic metronidazole and amoxycillin alone and combined. A placebo controlled study 2011 A prospective 12-month study of the effect of smoking cessation on periodontal clinical parameters J Clin Periodontol 38 6 562-571 Not RCT 2011 A prospective 12-month study of the effect of smoking cessation on periodontal clinical parameters J Clin Periodontol 38 6 562-71 Duplicate J Clin Periodontol 10 5 487-514 Duplicate J Clin Periodontol 10 5 487-514 Not commercially available J Clin Periodontol 28 11 1023-31 Retreatment per initial therapy J Periodontol 69 10 1148-54 Elyzol J Periodontol 76 11 1942-9 Not enough information on laser use parameters J Clin Periodontol 26 10 683-91 Doxy not adjunct 1983 1983 2001 1998 2005 1999 Microbiological and clinical effects of topical subgingival antimicrobial treatment on human periodontal disease Microbiological and clinical effects of topical subgingival antimicrobial treatment on human periodontal disease The use of PVP-iodine as an adjunct to nonsurgical treatment of chronic periodontitis Local metronidazole application in maintenance patients. Clinical and microbiological evaluation In vitro, clinical, and microbiological evaluation of a linear oscillating device for scaling and root planing Effects of smoking on local delivery of controlled-release doxycycline as compared to scaling and root planing Page 113 L. Wolinsky, R. Yukna, A. Polson, G. Carron and S. Garrett Sahrmann, P., M. A. Puhan, T. Attin and P. R. Schmidlin 2010 Systematic review on the effect of rinsing with povidone-iodine during nonsurgical periodontal therapy J Periodontal Res 45 2 153-164 Review Sakellari, D., I. D. Vouros, E. Aristodemou, A. B. Konstantinidis, S. Socransky and M. Goodson 2005 Tetracycline fibers as an adjunct in the treatment of nifedipine-induced gingival enlargement J Periodontol 76 6 1034-9 Not perio Sakellari, D., I. Vouros and A. Konstantinidis 2003 The use of tetracycline fibres in the treatment of generalised aggressive periodontitis: clinical and microbiological findings J Int Acad Periodontol 5 2 52-60 Aggressive perio Sakellari, D., I. Ioannidis, M. Antoniadou, T. Slini and A. Konstantinidis 2010 Clinical and microbiological effects of adjunctive, locally delivered chlorhexidine on patients with chronic periodontitis J Int Acad Periodontol 12 1 20-6 Duplicate of citation under Dimitra 2010 (included, CHX chip) 2008 Tetracycline gel as an adjunct to surgical root debridement Am J Dent 21 3 168-70 Surgical therapy 2002 Local antimicrobial therapy after initial periodontal treatment J Clin Periodontol 29 6 540-50 Head to head- no control J Periodontal Res 47 1 45-54 SRP delivery method J Periodontol 80 8 1237-1245 J Periodontol 80 8 1237-45 J Int Acad Periodontol 7 3 70-9 Not adjunct of interest J Int Acad Periodontol 5 4 106-15 Not adjunct of interest Sallum, A. W., R. Vasconcelos Alves, L. F. Teixeira Damis, P. F. Roesler Bertolini, F. H. Nociti Junior and E. A. Sallum Salvi, G. E., A. Mombelli, L. Mayfield, A. Rutar, J. Suvan, S. Garrett and N. P. Lang Santos, V. R., F. V. Ribeiro, J. A. Lima, T. S. Miranda, M. Feres, M. F. Bastos and P. M. Duarte Santos, V. R., J. A. Lima, A. C. De Mendonca, M. B. B. Maximo, M. Faveri and P. M. Duarte Santos, V. R., J. A. Lima, A. C. De Mendonca, M. B. Braz Maximo, M. Faveri and P. M. Duarte Sastravaha, G., G. Gassmann, P. Sangtherapitikul and W. D. Grimm Sastravaha, G., P. Yotnuengnit, P. Booncong and P. Sangtherapitikul Schlagenhauf, U., P. Stellwag and A. Fiedler Schwarz, F., A. Sculean, M. Berakdar, T. Georg, E. Reich and J. Becker July 2015 2012 2009 2009 2005 2003 Partial- and full-mouth scaling and root planing in type 2 diabetic subjects: a 12-mo follow-up of clinical parameters and levels of cytokines and osteoclastogenesis-related factors Effectiveness of full-mouth and partial-mouth scaling and root planing in treating chronic periodontitis in subjects with type 2 diabetes Effectiveness of full-mouth and partial-mouth scaling and root planing in treating chronic periodontitis in subjects with type 2 diabetes Adjunctive periodontal treatment with Centella asiatica and Punica granatum extracts in supportive periodontal therapy Adjunctive periodontal treatment with Centella asiatica and Punica granatum extracts. A preliminary study Duplicate Compared SRP done over 2 days to SRP by quadrant over 21 days. No control group with no SRP 1990 Subgingival irrigation in the maintenance phase of periodontal therapy J Clin Periodontol 17 9 650-3 Data in unusable format (% reduction from baseline) 2003 Clinical evaluation of an Er:YAG laser combined with scaling and root planing for non-surgical periodontal treatment. A controlled, prospective clinical study J Clin Periodontol 30 1 26-34 No adequate SRP only control Page 114 Schwarz, F., A. Sculean, M. Berakdar, T. Georg, E. Reich and J. Becker 2003 Schwarz, F., A. Sculean, T. Georg and E. Reich 2001 Schwarz, F., A. Sculean, M. Berakdar, T. Georg, E. Reich and J. Becker 2003 Schwarz, F., K. Bieling, S. Venghaus, A. Sculean, S. Jepsen and J. Becker 2006 Sculean, A., F. Schwarz, M. Berakdar, G. E. Romanos, M. Brecx, B. Willershausen and J. Becker Sculean, A., F. Schwarz, M. Berakdar, G. E. Romanos, N. B. Arweiler and J. Becker Serino, G., B. Rosling, P. Ramberg, M. K. Hellstrom, S. S. Socransky and J. Lindhe 2004 2004 2001 Sgolastra, F., A. Petrucci, R. Gatto, G. Marzo and A. Monaco 2011 Sgolastra, F., A. Petrucci, R. Gatto, M. Giannoni and A. Monaco 2011 Sgolastra, F., R. Gatto, A. Petrucci and A. Monaco 2012 Sgolastra, F., A. Petrucci, R. Gatto and A. Monaco 2012 Sgolastra, F., A. Petrucci, R. Gatto and A. Monaco 2012 Shar, A. and A. R. Pradeep 2012 Sharma, A. and A. R. Pradeep 2012 July 2015 Clinical evaluation of an Er:YAG laser combined with scaling and root planing for non-surgical periodontal treatment. A controlled, prospective clinical study Periodontal treatment with an Er: YAG laser compared to scaling and root planing. A controlled clinical study Periodontal treatment with an Er:YAG laser or scaling and root planing. A 2-year follow-up split-mouth study Influence of fluorescence-controlled Er:YAG laser radiation, the Vector system and hand instruments on periodontally diseased root surfaces in vivo Non-surgical periodontal treatment with a new ultrasonic device (Vector-ultrasonic system) or hand instruments Periodontal treatment with an Er:YAG laser compared to ultrasonic instrumentation: a pilot study The effect of systemic antibiotics in the treatment of patients with recurrent periodontitis Photodynamic therapy in the treatment of chronic periodontitis: a systematic review and meta-analysis Long-term efficacy of subantimicrobial-dose doxycycline as an adjunctive treatment to scaling and root planing: a systematic review and meta-analysis Effectiveness of systemic amoxicillin/metronidazole as adjunctive therapy to scaling and root planing in the treatment of chronic periodontitis: a systematic review and meta-analysis Effectiveness of systemic amoxicillin/metronidazole as an adjunctive therapy to full-mouth scaling and root planing in the treatment of aggressive periodontitis: a systematic review and meta-analysis Efficacy of Er:YAG laser in the treatment of chronic periodontitis: systematic review and meta-analysis Clinical efficacy of 1% Alendronate gel in adjunct to mechanotherapy in the treatment of aggressive periodontitis: A randomized controlled clinical trial Clinical efficacy of 1% alendronate gel in adjunct to mechanotherapy in the treatment of J Clin Periodontol 30 1 26-34 Duplicate J Periodontol 72 3 361-7 Laser not as adjunct J Periodontol 74 5 590-6 Laser not as adjunct J Clin Periodontol 33 3 200-8 No control group J Clin Periodontol 31 6 428-33 SRP delivery method J Periodontol 75 7 966-73 Laser not used as adjunct J Clin Periodontol 28 5 411-8 Not RCT Lasers Med Sci Review J Periodontol 82 11 1570-81 Review J Periodontol 83 10 1257-69 Review J Periodontol 83 6 731-43 Review Lasers Med Sci 27 3 661-73 Review J Periodontol 83 1 19-26 Duplicate and incorrect reference J Periodontol 83 1 19-26 Aggressive perio Page 115 Sharma, A. and A. R. Pradeep 2012 Shiloah, J. and M. R. Patters 1996 Shiloah, J. and M. R. Patters 1996 Sigusch, B. W., A. Guntsch, A. Pfitzner and E. Glockmann 2005 Sigusch, B. W., A. Pfitzner, T. Nietzsch and E. Glockmann 2005 Sigusch, B. W., A. Pfitzner, T. Nietzsch and E. Glockmann 2005 Sigusch, B., M. Beier, G. Klinger, W. Pfister and E. Glockmann 2001 Silva, M. P., M. Feres, T. A. Oliveira Sirotto, G. M. Silva Soares, J. A. Velloso Mendes, M. Faveri and L. C. Figueiredo 2011 Silva, M. P., M. Feres, T. A. Sirotto, G. M. Soares, J. A. Mendes, M. Faveri and L. C. Figueiredo 2011 Slot, D. E., T. J. Koster, S. Paraskevas and G. A. Van der Weijden Slot, D. E., A. A. Kranendonk, S. Paraskevas and F. Van der Weijden aggressive periodontitis: a randomized controlled clinical trial Clinical efficacy of 1% alendronate gel as a local drug delivery system in the treatment of chronic periodontitis: a randomized, controlled clinical trial Repopulation of periodontal pockets by microbial pathogens in the absence of supportive therapy Repopulation of periodontal pockets by microbial pathogens in the absence of supportive therapy Enhanced root planing and systemic metronidazole administration improve clinical and microbiological outcomes in a two-step treatment procedure Periodontal dressing (Vocopac) influences outcomes in a two-step treatment procedure Periodontal dressing (Vocopac(registered trademark)) influences outcomes in a two-step treatment procedure A 2-step non-surgical procedure and systemic antibiotics in the treatment of rapidly progressive periodontitis Clinical and microbiological benefits of metronidazole alone or with amoxicillin as adjuncts in the treatment of chronic periodontitis: A randomized placebo-controlled clinical trial Clinical and microbiological benefits of metronidazole alone or with amoxicillin as adjuncts in the treatment of chronic periodontitis: a randomized placebo-controlled clinical trial J Periodontol 83 1 8-Nov Not commercially available J Periodontol 67 2 130-9 CAL data not separated by treatment J Periodontol 67 2 130-9 Duplicate J Periodontol 76 6 991-7 Aggressive perio J Clin Periodontol 32 4 401-5 Duplicate J Clin Periodontol 32 4 401-405 Aggressive perio J Periodontol 72 3 275-83 Not only SRP includes enhanced root planing J Clin Periodontol 38 9 828-837 Not 6 month trial J Clin Periodontol 38 9 828-37 Duplicate 2008 The effect of the Vector scaler system on human teeth: a systematic review Int J Dent Hyg 6 3 154-65 Review 2009 The effect of a pulsed Nd:YAG laser in nonsurgical periodontal therapy J Periodontol 80 7 1041-56 Review Smith, S. R., D. M. Foyle, J. Daniels, S. Joyston-Bechal, F. C. Smales, A. Sefton and J. Williams 2002 A double-blind placebo-controlled trial of azithromycin as an adjunct to non-surgical treatment of periodontitis in adults: clinical results J Clin Periodontol 29 1 54-61 No CAL data Soder, P. O., L. Frithiof, S. Wikner, F. Wouters, P. E. Engstrom, B. Rubin, U. Nedlich and B. Soder 1990 The effect of systemic metronidazole after non-surgical treatment in moderate and advanced periodontitis in young adults J Periodontol 61 5 281-8 No CAL data Soder, B., U. Nedlich and L. J. Jin 1999 Longitudinal effect of non-surgical treatment and systemic metronidazole for 1 week in J Periodontol 70 7 761-71 No 1 year data July 2015 Page 116 smokers and non-smokers with refractory periodontitis: a 5-year study Soskolne, W. A., P. A. Heasman, A. Stabholz, G. J. Smart, M. Palmer, M. Flashner and H. N. Newman 1997 Soskolne, W. A., H. M. Proskin and A. Stabholz 2003 Stabholz, A., W. A. Soskolne, M. Friedman and M. N. Sela 1991 Stabholz, A., L. Shapira, D. Mahler, Y. Gellman, T. Ramon, E. Dolev, M. Schwartz, L. Berger, H. M. Proskin, R. D. Finkelman, M. Flashner, B. Kolatch and A. Soskolne Stelzel, M. and L. Flores-deJacoby Swierkot, K., C. I. Nonnenmacher, R. Mutters, L. Flores-de-Jacoby and R. Mengel Sustained local delivery of chlorhexidine in the treatment of periodontitis: a multi-center study Probing depth changes following 2 years of periodontal maintenance therapy including adjunctive controlled release of chlorhexidine The use of sustained release delivery of chlorhexidine for the maintenance of periodontal pockets: 2-year clinical trial J Periodontol 68 1 32-8 Retreatment with chip at 3 months in select pockets J Periodontol 74 4 420-7 Not RCT J Periodontol 62 7 429-33 Not commercially available 2000 Using the PerioChip in treating adult periodontitis: an interim report Compend Contin Educ Dent 21 4 325-8, 330, 332 passim; quiz 338 2000 Topical metronidazole application as an adjunct to scaling and root planing J Clin Periodontol 27 6 447-52 Not available in US 2009 One-stage full-mouth disinfection versus quadrant and full-mouth root planing J Clin Periodontol 36 3 240-9 CHX gel not avail US Taggart, J. A., R. M. Palmer and R. F. Wilson 1990 A clinical and microbiological comparison of the effects of water and 0.02% chlorhexidine as coolants during ultrasonic scaling and root planing J Clin Periodontol 17 1 32-7 CHX as coolant Timmerman, M. F., G. A. van der Weijden, T. J. van Steenbergen, M. S. Mantel, J. de Graaff and U. van der Velden 1996 Evaluation of the long-term efficacy and safety of locally-applied minocycline in adult periodontitis patients J Clin Periodontol 23 8 707-16 Not commercially available Tomasi, C., T. Koutouzis and J. L. Wennstrom 2008 J Periodontol 79 3 431-9 Not SRP (debridement) Tomasi, C. and J. L. Wennstrom 2011 J Periodontol 82 2 210-8 Not SRP (debridement) Tonetti, M. S., P. Cortellini, G. Carnevale, M. Cattabriga, M. de Sanctis and G. P. Pini Prato 1998 J Clin Periodontol 25 9 728-36 Not available US Tonetti, M. S., N. P. Lang, P. Cortellini, J. E. Suvan, P. Eickholz, I. Fourmousis, H. Topoll, T. Vangsted and B. Wallkamm 2012 J Clin Periodontol 39 5 475-82 14% not commercially available July 2015 Locally delivered doxycycline as an adjunct to mechanical debridement at retreatment of periodontal pockets Locally delivered doxycycline as an adjunct to mechanical debridement at retreatment of periodontal pockets: outcome at furcation sites A controlled multicenter study of adjunctive use of tetracycline periodontal fibers in mandibular class II furcations with persistent bleeding Effects of a single topical doxycycline administration adjunctive to mechanical debridement in patients with persistent/recurrent periodontitis but acceptable oral hygiene during supportive periodontal therapy Not RCT Page 117 Tunkel, J., A. Heinecke and T. F. Flemmig 2002 Unsal, E., M. Akkaya and T. F. Walsh 1994 Valerio Lopes, B. M., L. H. Theodoro, R. F. Melo, G. M. De Azevedo Thompson and R. A. Chierici Marcantonio Vandekerckhove, B. N., M. Quirynen and D. van Steenberghe Vandekerckhove, B. N., C. M. Bollen, C. Dekeyser, P. Darius and M. Quirynen Van der Weijden, G. A. and M. F. Timmerman Van Steenberghe, D., B. Rosling, P. O. Soder, R. G. Landry, U. Van Der Velden, M. F. T. Timmerman, E. F. McCarthy, G. Vandenhoven, C. Wouters, M. Wilson, J. Matthews and H. N. Newman Varela, V. M., D. Heller, M. X. Silva-Senem, M. C. M. B. Torres, A. P. V. Colombo and E. J. FeresFilho Varela, V. M., D. Heller, M. X. Silva-Senem, M. C. Torres, A. P. Colombo and E. J. Feres-Filho Walker, C. B., J. M. Gordon, I. Magnusson and W. B. Clark Wennstrom, J. L., H. N. Newman, S. R. MacNeill, W. J. Killoy, G. S. Griffiths, D. G. Gillam, L. Krok, I. G. Needleman, G. Weiss and S. Garrett 2010 1997 1996 2002 1999 2011 2011 1993 A systematic review of efficacy of machinedriven and manual subgingival debridement in the treatment of chronic periodontitis Influence of a single application of subgingival chlorhexidine gel or tetracycline paste on the clinical parameters of adult periodontitis patients Clinical and microbiologic follow-up evaluations after non-surgical periodontal treatment with erbium:YAG laser and scaling and root planing The use of tetracycline-containing controlledrelease fibers in the treatment of refractory periodontitis Full- versus partial-mouth disinfection in the treatment of periodontal infections. Long-term clinical observations of a pilot study A systematic review on the clinical efficacy of subgingival debridement in the treatment of chronic periodontitis A 15-month evaluation of the effects of repeated subgingival minocycline in chronic adult periodontitis Systemic antimicrobials adjunctive to a repeated mechanical and antiseptic therapy for aggressive periodontitis: A 6-month randomized controlled trial Systemic antimicrobials adjunctive to a repeated mechanical and antiseptic therapy for aggressive periodontitis: a 6-month randomized controlled trial A role for antibiotics in the treatment of refractory periodontitis 72-81; discussion 90-1 J Clin Periodontol 29 Suppl 3 J Clin Periodontol 21 5 351-5 J Periodontol 81 5 682-691 Duplicate citation of included article Lopes J Periodontol 68 4 353-61 Not RCT J Periodontol 67 12 1251-9 CHX gel not avail US J Clin Periodontol 55-71; discussion 90-1 29 Suppl 3 Review Not 6 month trial Review J Periodontol 70 6 657-667 Not commercially available J Periodontol 82 8 1121-1130 J Periodontol 82 8 1121-30 Duplicate J Periodontol 64 8 Suppl 772-81 Review Aggressive perio 2001 Utilisation of locally delivered doxycycline in non-surgical treatment of chronic periodontitis. A comparative multi-centre trial of 2 treatment approaches J Clin Periodontol 28 8 753-61 Control group different from experimental group Wennstrom, J. L., L. Heijl, G. Dahlen and K. Grondahl 1987 Periodic subgingival antimicrobial irrigation of periodontal pockets (I). Clinical observations J Clin Periodontol 14 9 541-50 For SRP part of trial, not long enough (20 weeks) Westfelt, E., H. Rylander, G. Dahlen and J. Lindhe 1998 J Clin Periodontol 25 7 536-41 Not randomized Williams, R. C., D. W. Paquette, S. Offenbacher, D. F. Adams, G. C. Armitage, K. Bray, J. Caton, D. 2001 J Periodontol 72 11 1535-44 No CAL data (ARESTIN) July 2015 The effect of supragingival plaque control on the progression of advanced periodontal disease Treatment of periodontitis by local administration of minocycline microspheres: a controlled trial Page 118 L. Cochran, C. H. Drisko, J. P. Fiorellini, W. V. Giannobile, S. Grossi, D. M. Guerrero, G. K. Johnson, I. B. Lamster, I. Magnusson, R. J. Oringer, G. R. Persson, T. E. Van Dyke, L. F. Wolff, E. A. Santucci, B. E. Rodda and J. Lessem Wilson T.G, Jr., M. K. McGuire, G. Greenstein and M. Nunn 1997 Wilson, T. G., Jr., M. K. McGuire, G. Greenstein and M. Nunn 1997 Winkel, E. G., A. J. Van Winkelhoff, M. F. Timmerman, T. Vangsted and U. Van der Velden 1997 Wong, M. Y., C. L. Lu, C. M. Liu, L. T. Hou and W. K. Chang 1998 Worthington, H. and I. Needleman Xajigeorgiou, C., D. Sakellari, T. Slini, A. Baka and A. Konstantinidis Yamaoka, M., T. Uematsu, T. Shiba, T. Matsuura, Y. Ono, M. Ishizuka, H. Naramoto, M. Takahashi, M. Sugiura-Tomita, K. Iguchi, S. Yamashita and K. Furusawa Yek, E. C., S. Cintan, N. Topcuoglu, G. Kulekci, H. Issever and A. Kantarci Yen, C. A., P. D. Damoulis, P. C. Stark, P. L. Hibberd, M. Singh and A. S. Papas 2005 2006 J Periodontol 68 11 1029-1032 Not commercially available J Periodontol 68 11 1029-32 Duplicate J Clin Periodontol 24 8 573-9 Not RCT J Formos Med Assoc 97 7 490-7 Not commercially available Periodontol 2000 37 J Clin Periodontol 33 11-Sep Not a trial 4 254-64 Aggressive perio 2008 Effect of inorganic polyphosphate in periodontitis in the elderly Gerodontology 25 1 7-Oct 2010 Efficacy of amoxicillin and metronidazole combination for the management of generalized aggressive periodontitis J Periodontol 81 7 964-74 Aggressive perio 2008 The effect of a selective cyclooxygenase-2 inhibitor (celecoxib) on chronic periodontitis J Periodontol 79 1 104-13 Not adjunct of interest Photomed Laser Surg 30 6 325-30 Not 6 month trial Yilmaz, S., B. Kut, H. Gursoy, B. Eren-Kuru, U. Noyan and T. Kadir 2012 Zandbergen, D., D. E. Slot, C. M. Cobb and F. A. Van der Weijden 2012 Zee, K. Y., D. H. Lee and E. F. Corbet 2006 July 2015 Tetracycline fibers plus scaling and root planing versus scaling and root planning alone: Similar results after 5 years Tetracycline fibers plus scaling and root planing versus scaling and root planing alone: similar results after 5 years Effects of metronidazole in patients with "refractory" periodontitis associated with Bacteroides forsythus Clinical response of localized recurrent periodontitis treated with scaling, root planing, and tetracycline fiber Evidence-based periodontal disease prevention and treatment: introduction Clinical and microbiological effects of different antimicrobials on generalized aggressive periodontitis Er:YAG laser versus systemic metronidazole as an adjunct to nonsurgical periodontal therapy: a clinical and microbiological study The Clinical Effect of Scaling and Root Planing and The Concomitant Administration of Systemic Amoxicillin and Metronidazole: A Systematic Review Repeated oral hygiene instructions alone, or in combination with metronidazole dental gel with or without subgingival scaling in adult periodontitis patients: a one-year clinical study J Periodontol J Int Acad Periodontol Not commercially available Review 8 4 125-35 Subgingival scaling - no root planing - possible cross over effect from gel arm Page 119 Zingale, J., L. Harpenau, G. Bruce, D. Chambers and W. Lundergan 2012 The effectiveness of scaling and root planing with adjunctive time-release minocycline using an open and closed approach for the treatment of periodontitis Gen Dent 60 4 300-5 No CAL data Excluded studies and reasons for exclusion – updated literature search Author Year Ahamed, S., Jalaluddin, M., Khalid, I., Moon, N., Shaf, T. K. and Ali, F. M. 2013 Balata, M. L., Andrade, L. P., Santos, D. B., Cavalcanti, A. N., Tunes Uda, R., Ribeiro Edel, P. and Bittencourt, S. 2013 Dilsiz, A., Canakci, V. and Aydin, T. 2013 Dilsiz, A. and Sevinc, S. 2014 Engebretson, S. P., Hyman, L. G., Michalowicz, B. S., Schoenfeld, E. R., Gelato, M. C., Hou, W., Seaquist, E. R., Reddy, M. S., Lewis, C. E., Oates, T. W., Tripathy, D., Katancik, J. A., Orlander, P. R., Paquette, D. W., Hanson, N. Q. and Tsai, M. Y. Euzebio Alves, V. T., de Andrade, A. K., Toaliar, J. M., Conde, M. July 2015 Title The use of controlled release locally delivered 10% doxycycline hyclate gel as an adjunct to scaling and root planing in the treatment of chronic periodontitis: clinical and microbiological results Photodynamic therapy associated with fullmouth ultrasonic debridement in the treatment of severe chronic periodontitis: a randomizedcontrolled clinical trial Clinical effects of potassium-titanyl-phosphate laser and photodynamic therapy on outcomes of treatment of chronic periodontitis: a randomized controlled clinical trial KTP laser therapy as an adjunctive to scaling and root planing in treatment of chronic periodontitis Journal Volume Page Reason if Exclude J Contemp Dent Pract 14 1080-6 Not an RCT J Appl Oral Sci 21 208-14 Debridement (no root planing) J Periodontol 84 278-86 Duplicate with Dilsiz 2012 (already included) KTP laser not available in US Acta Odontol Scand 2013 The effect of nonsurgical periodontal therapy on hemoglobin A1c levels in persons with type 2 diabetes and chronic periodontitis: a randomized clinical trial 310 2523-32 include SRP retreated at all sites at 3 months (Question 1) 2013 Clinical and microbiological evaluation of high intensity diode laser adjutant to non-surgical periodontal treatment: a 6-month clinical trial Clin Oral Investig 17 87-95 Duplicate with Alves 2012 (already included) Page 120 C., Zezell, D. M., Cai, S., Pannuti, C. M. and De Micheli, G. Feres, M., Soares, G. M., Mendes, J. A., Silva, M. P., Faveri, M., Teles, R., Socransky, S. S. and Figueiredo, L. C. Fiorini, T., Susin, C., da Rocha, J. M., Weidlich, P., Vianna, P., Moreira, C. H., Bogo Chies, J. A., Rosing, C. K. and Oppermann, R. V. Franco, E. J., Pogue, R. E., Sakamoto, L. H., Cavalcante, L. L., Carvalho, D. R. and de Andrade, R. V. Giannopoulou, C., Cappuyns, I., Cancela, J., Cionca, N. and Mombelli, A. Gomes, S. C., Romagna, R., Rossi, V., Corvello, P. C. and Angst, P. D. Jain, M., Dave, D., Jain, P., Manohar, B., Yadav, B. and Shetty, N. Kapellas, K., Maple-Brown, L. J., Bartold, P. M., Brown, A., O'Dea, K., Slade, G. D., Celermajer, D. S., Jamieson, L. M. and Skilton, M. R. Kondreddy, K., Ambalavanan, N., Ramakrishna, T. and Kumar, R. S. Kucukcoskun, M., Baser, U., Oztekin, G., Kiyan, E. and Yalcin, F. Kumar, A. K., Reddy, N. R., Babu, M., Kumar, P. M., Reddy, V. S. and Chavan, C. V. July 2015 1149-58 1/2 of intervention group also had CHX rinse. Data not stratefied between groups that had CHX and groups that did not. 2012 Metronidazole alone or with amoxicillin as adjuncts to non-surgical treatment of chronic periodontitis: a 1-year double-blinded, placebo-controlled, randomized clinical trial 2013 Effect of nonsurgical periodontal therapy on serum and gingival crevicular fluid cytokine levels during pregnancy and postpartum 2014 Increased expression of genes after periodontal treatment with photodynamic therapy 2012 Effect of photodynamic therapy, diode laser, and deep scaling on cytokine and acutephase protein levels in gingival crevicular fluid of residual periodontal pockets J Periodontol 83 2014 Supragingival treatment as an aid to reduce subgingival needs: a 450-day investigation Braz Oral Res 28 2013 Efficacy of xanthan based chlorhexidine gel as an adjunct to scaling and root planing in treatment of the chronic periodontitis J Indian Soc Periodontol 17 2014 Effect of a periodontal intervention on pulse wave velocity in Indigenous Australians with periodontal disease: The PerioCardio randomized controlled trial 9 e44 Publication type (abstract) 16 553-7 Not an RCT J Periodontol 84 863-70 Not randomized Contemp Clin Dent 4 303-6 Less than 6 months (8 weeks) 2012 2013 2013 Effectiveness of a controlled release chlorhexidine chip (PerioCol-CG) as an adjunctive to scaling and root planing when compared to scaling and root planing alone in the treatment of chronic periodontitis: A comparative study Initial periodontal treatment for prevention of chronic obstructive pulmonary disease exacerbations Estimation of prostaglandin E2 levels in gingival crevicular fluid in periodontal health, disease and after treatment J Clin Periodontol 39 48 126-33 unsure Less than 6 months (calculated to be 23 weeks); also, mean CAL not reported Photodiagnosis Photodyn Ther 11 41-7 Less than 6 months; not an RCT 1018-27 No CAL data; Other treatment (DSL and PDT) are not in conjunction with SRP no SRP-nly control 439-43 Chlosite gel not available in US Page 121 Martinez, G. L., Koury, J. C., Brito, F., Fischer, R. G., Gustafsson, A. and Figueredo, C. M. Matesanz, P., Herrera, D., Echeverria, A., O'Connor, A., Gonzalez, I. and Sanz, M. Meulman, T., Giorgetti, A. P., Gimenes, J., Casarin, R. C., Peruzzo, D. C. and Nociti, F. H., Jr. 2014 2013 2013 Miremadi, S. R., De Bruyn, H., Steyaert, H., Princen, K., Sabzevar, M. M. and Cosyn, J. 2014 Muller Campanile, V. S., Giannopoulou, C., Campanile, G., Cancela, J. A. and Mombelli, A. 2013 Pera, C., Ueda, P., Casarin, R. C., Ribeiro, F. V., Pimentel, S. P., Casati, M. Z. and Cirano, F. R. 2012 Petelin, M., Perkic, K., Seme, K. and Gaspirc, B. 2014 Pradeep, A. R., Bajaj, P., Agarwal, E., Rao, N. S., Naik, S. B., Kalra, N. and Priyanka, N. 2013 Pradeep, A. R., Singh, S. P., Martande, S. S., Naik, S. B., N, P., Kalra, N. and Suke, D. K. 2014 Putt, M. S. and Proskin, H. M. 2013 Santos, V. R., Lima, J. A., Miranda, T. S., Goncalves, T. E., Figueiredo, L. C., Faveri, M. and Duarte, P. M. 2013 July 2015 The impact of non-surgical periodontal treatment on serum levels of long chainpolyunsaturated fatty acids: a pilot randomized clinical trial A randomized clinical trial on the clinical and microbiological efficacy of a xanthan gel with chlorhexidine for subgingival use One stage, full-mouth, ultrasonic debridement in the treatment of severe chronic periodontitis in smokers: a preliminary, blind and randomized clinical trial A randomized controlled trial on immediate surgery versus root planing in patients with advanced periodontal disease: a costeffectiveness analysis Single or repeated antimicrobial photodynamic therapy as adjunct to ultrasonic debridement in residual periodontal pockets: clinical, microbiological, and local biological effects Double-masked randomized clinical trial evaluating the effect of a triclosan/copolymer dentifrice on periodontal healing after onestage full-mouth debridement Effect of repeated adjunctive antimicrobial photodynamic therapy on subgingival periodontal pathogens in the treatment of chronic periodontitis Local drug delivery of 0.5% azithromycin in the treatment of chronic periodontitis among smokers Clinical and microbiological effects of levofloxacin in the treatment of chronic periodontitis: a randomized, placebocontrolled clinical trial Custom tray application of peroxide gel as an adjunct to scaling and root planing in the treatment of periodontitis: results of a randomized controlled trial after six months Full-mouth disinfection as a therapeutic protocol for type-2 diabetic subjects with chronic periodontitis: twelve-month clinical outcomes: a randomized controlled clinical trial J Periodontal Res 49 268-74 Less than 6 months (4 months) Clin Oral Investig 17 55-66 Chlosite gel not available in US J Int Acad Periodontol 15 83-90 Data only in graph and cannot be abstracted J Clin Periodontol 41 164-71 Compared SRP to surgery 8-Jan Debridement (no root planing) 909-16 Debridement (no root planing) AND not medical adjunct (OTC) Lasers in Medical Science J Periodontol 83 experitmental tx with multiple PDT episodes; improper control (scaler) Lasers Med Sci Aust Dent J 58 34-40 J Investig Clin Dent Experimental gel (researcher-prepared) Experimental therapy J Clin Dent 24 100-7 No CAL data J Clin Periodontol 40 155-62 CHX gel not available in US Page 122 Shiloah, J., Bland, P. S., Scarbecz, M., Patters, M. R., Stein, S. H. and Tipton, D. A. 2014 Slot, D. E., Timmerman, M. F., Versteeg, P. A., van der Velden, U. and van der Weijden, F. A. 2012 Tuter, G., Ozdemir, B., Kurtis, B., Serdar, M., Yucel, A. A. and Ayhan, E. 2013 Wehmeyer, M. M., Kshirsagar, A. V., Barros, S. P., Beck, J. D., Moss, K. L., Preisser, J. S. and Offenbacher, S. 2013 July 2015 The effect of long-term aspirin intake on the outcome of non-surgical periodontal therapy in smokers: a double-blind, randomized pilot study Adjunctive clinical effect of a water-cooled Nd:YAG laser in a periodontal maintenance care programme: a randomized controlled trial Short term effects of non-surgical periodontal treatment on gingival crevicular fluid levels of tissue plasminogen activator (t-PA) and plasminogen activator inhibitor 2 (PAI-2) in patients with chronic and aggressive periodontitis A randomized controlled trial of intensive periodontal therapy on metabolic and inflammatory markers in patients With ESRD: results of an exploratory study J Periodontal Res 49 102-9 OTC J Clin Periodontol 39 1159-65 No CAL data Arch Oral Biol 58 391-6 Less than 6 months (6 weeks) and not an RCT Am J Kidney Dis 61 450-8 Treatment is SRP+adjunct vs no tx (not vs SRP alone) Page 123 Appendix 3 – Study characteristics, outcomes data, and critical appraisals of individual trials Scaling and root planing – Study characteristics Citation: Author, Year Country Special population? (e.g. smokers) and other data regarding inclusion - exclusion criteria for patients and teeth Severity of disease (e.g. refractory, mild, moderate) Control Test subject subject age age (mean, (mean, median, range median, range as described) as described) Control Control Dose/ duration/ frequency/ timing if applicable Test (typically adjunct) Test (typically adjunct) Dose/ duration/ frequency/ timing including simultaneous/ before/ after Was standard counseling mentioned as part of control treatment? Trial design (split mouth / parallel group / cross over) Duration of study Adverse events reported Berglundh, 1998 Sweden no mention of cigarettes or any health condition advanced periodontal disease 35-58 years no subgingival treatment and placebo Placebo not described SRP+placebo local anesthesia 3-5 sessions unspecified operator / placebo not described oral hygiene instruction and one session of supragingival scaling. The oral hygiene instruction was, if indicated, reinforced during the course of the trial. Split mouth for these data (Factorial) 12 months NR Chen 2012 China Adults with type 2 diabetes aged 38 to 81; with a ≥ 1 mm mean clinical attachment loss ([AL]; including slight, moderate, and severe periodontitis Chronic periodontitis Group 3. No TX or OHI n/a Group 1: SRP at baseline and subgingival debridement at 3 months. Group 2: SRP at baseline and supragingival prophy at 3 months no. Control group specifically received no OHI Parallel group 6 months no patients reported adverse events Parallel group 6 months Group 1: 59.86 ± 9.48 Group 2: 57.91 ± 11.35 Group 1: SRP at baseline and subgingival debridement at 3 months. 63.2 ± 8.51 Group 2: SRP at baseline and supragingival prophy at 3 months No Jones 1994 Q1 only Note: data read from figure; only max whisker value plotted in figure U.S. presence of at least two sites on different teeth in one quadrant, excluding third molars, that had probing depths of 7 mm or greater. In addition, the subgingival presence by anaerobic culture of one or more of the following pathogens had to be demonstrated at these sites before the Moderate to advanced adult subject was included: Porphyromonas periodontitis gingivalis, Prevotella intermedia, and/or Actinobacillus actino- mycetemcomitans / not pregnant or lactating / no chronic systemic diseases / no antibiotic prophy for dental tx / systemic antibiotics or continuous non-steroidal antiinflammatory drugs within the previous 3 months or chronic low-dose tetracycline 51 patients (age range 28 to 68) were randomized into one of four treatment groups, no info on specific groups No treatment No treatment (NoTx) in which no scaling and root planing or subgingival minocycline was utilized at any site in any quadrant. A supragingival irrigation with a sterile saline placebo was applied by means of an irrigating syringe in the study quadrant for approximately 30 seconds in an attempt to create patient blinding. / The nonstudy quadrants received no supragingival or subgingival treatment during the course of the experiment and thus each patient received only one treatment type. 47 ±9 (range 35-65) Group 4: supragingival coronal polish single episode by hygienist No treatment N/A SRP only Scaling and root planing only (SRP) in which scaling and, root planing was accomplished on all teeth in the study quadrant. / A supragingival irrigation with a sterile saline placebo was applied by means of an A standardized oral hygiene irrigating syringe in the study regimen was presented to quadrant for approximately 30 each patient at the beginning of seconds in an attempt to create the study, but no professional patient blinding. No treatment supragingival cleaning was was performed in the other performed except for scaling and quadrants / The nonstudy root planing as indicated above. quadrants received no supragingival or subgingival treatment during the course of the experiment and thus each patient received only one treatment type. AE information was not reported Unclear as to exact reason. 51 started the study and 39 completed with evaluable data sets. Any site which had undergone a 2 mm or greater loss of relative clinical attachment as compared to baseline was considered to be a progressing Group 2: SRP by a hygienist; Kahl, 2007 Lindhe 1983 Germany Sweden Neill 1997 (SRP vs no tx) DATA IN FIGURE AND TEXT INCONSISTENT no exclusion criteria, included smokers 8 of 20 subjects no moderate to advanced chronic perio (5-8mm) advanced periodontal disease. At least 20 remaining teeth and 4 pairs of diseased sites around 37-52 years of contralateral premolars and 37-52 years of age age incisors where pockets could be probed to ≥6mm and where ≥40% of the alveolar bone had been lost [Group 3: SRP by a dentist; data not being used] SRP No U.S. probing depths > 4 mm with radiographic bone loss moderate to severe adult periodontitis (PD 4mm or greater) average age of 44 years (range 3353) No treatment No treatment SRP Generalized Periodontitis (50% bone loss) range:32-72 No treatment + placebo Ultrasonic and hand instruments SRP+placebo single episode yes split mouth 6 months Did not seek or report adverse events Once by quadrant yes Split mouth 50 weeks Did not seek or report adverse events 6 months None reported; minimal post-op pain reported Split mouth 24 weeks Did not seek or report adverse events Parallel group 6 months Patient perceptions on pain, analgesics, body temp, oral ulcerations & other adverse events were collected via VAS questionnaire at the end of treatment (after 4th quad; after debridement). RESULTS: no differences between groups; none reported acute problems. Mechanical SRP was used to remove calculus and other Split mouth deposits from the root surfaces Yes; but only mentioned for no to achieve the endoint of a quadrants treatment group (not SRP smoot, glass-like root surface. randomly group); patient plaque control Ultrasonic instrumentation was assigned to one of instructions included where appropriate, and three experimental the site was irrigated with a groups: sterile saline solution. 15 current smokers, 17 non-smokers, not asked about past smoking Ng, 1998 Ribeiro (DEL PELOSO), 2008 USA Brazil Thirty-two subjects (18 males and 14 females, ranging in age from 32 to 72 years) with generalized Periodontitis were selected according to the following criteria: 1) radiographic bone loss greater than 50% in at least 2 matching tooth types per subject and 2) periodontal probing depth ≥5 mm in 1 or more sites in at least 2 matching tooth types. Inclusion: Non-smokers only; CAL ≥5mm; 8 teeth with pocket depth ≥5 mm, 2 of which ≥7mm and 2 ≥6mm; Exclusion: no perio tx or antibiotics for previous 6 months; no systemic pathogies; no pregnant women; no medical disorders req prophy antibiotics or drugs known to affect perio status; no ortho; Severe chronic periodontitis Mean = 45.5 (range 30-66 years) Mean = 38.9 (range 33-55 years) SRP (quadrant) 12 of 27 smokers Rotundo 2010 (SRP vs no tx) Italy 2 teeth per quad with PD 4-9 mm with BOP; presence of at least one incisor, premolar and molar per quad Moderate to severe chronic 50.5 ± 11.7 years periodontitis 50.5 ± 11.7 years Supragingival prophy at the end of the study No Van Dyke 2002 U.S. at least two teeth having one site each with pocket depth (PD) >=6mm and with prostaglandin E2 (PG E2) levels > 66.2ng/ml in gingival crevicular fluid. Moderate to severe periodontitis Not stated Not stated No tx Prior to start of study, supra calculus removed for all subjects, Test and Control; Quadrant scaling with 1 week interval between Quad scaling with ultrasonic and gracey currets; no time limit for appointment; local anesthesia used as necessary; Monthly: maintenance program of professional supraging plaque contol and reinforcement of OHI; Retreatment at 3 months for PPD ≥5mm and BOP One appointment / using mechanical instruments (ultrasonics, hand instruments, polishing) was performed. For ethical reasons, this control quadrant was treated at the end of the study as needed For those patients receiving no Tx or SRP only irrigation of ,the surrounding test area was performed using sterile wvater or saline to ensure patient blinding. Perio debridement (one session) SRP SRP received placebo capsules via oral administration (1 capsule per day). One session of full-mouth periodontal debridement using an ultrasonic scaler (Profi III). Specific tips were used (33 Probe, Amdent, Stockholm, Sweden). 45 minute time limit; local anesthesia used as necessary. Monthly: maintenance program of professional supraging plaque contol and reinforcement of OHI; Re-treatment at 3 months for PPD ≥5mm and BOP no All patients initially received detailed information on the etiology of period disease and instructions for proper, selfperformed plaque control measures, including inter-dental cleaning with dental floss and inter-dental tooth- brushes. In the initial sessions, patients also had plaque retentive factors (caries, excess of restorations and supragingival calculus) removed. The baseline mea- surements were done 30 days after this initial phase. One appointment / Gracey’s curettes and an ultrasonic device (Mini- Piezons, EMS Electro Medical Systems S.A., Nyon, Switzerland) were used. For all treatments, the instrumentation was carried out until the operator felt a planed Split mouth; Each and well-debrided dental strategy was OHI for all patients as well as full surface. Subgingival root randomly mouth supragingival prophy planing and/or laser application assigned and using ultrasonic / hand were performed in the sites with performed in instruments a periodontal PDX4 mm. The one of the four sites with a PDo4mm were quadrants treated using ultrasonic device and hand instrument/ polishing. A 60-s rinse with 0.12% chlorhexidine digluconate was prescribed twice a day for 1 week. Patients were recalled for control and supportive For those patients receiving no Tx or SRP only irrigation of ,the surrounding test area was performed using sterile wvater or saline to ensure patient blinding. No Parallel Complications: 5 perio abscesses reported, none in the SRP alone group; 1 pt reported fever during the 1st week post-tx immediately at the end of treatment, the only statistically significant difference resulted between SRP and S ( p50.0132) (Table 3). 6 months 6 months After 1 week, no difference was observed for pain ( p=0.5968) and chewing discomfort ( p=0.3151) between the four types of treatments. However, the SRP procedure caused a higher dental hypersensitivity than the S treatment (p50.0294) (Table 3). 6-months: Regarding the patient-centred analysis based on the questionnaire, the variables pain (p=0.4732), dental hypersensitivity (p=0.3135) and chewing discomfort (p=0.9574), did not show any statistically significant difference among the procedures (Table 3). An SRP + MPTS patient experienced two adverse events (AEs) - a black hairy tongue ruled to be possibly dtug related by the investigator and abscess formation at study sites with oedema, which was not considered to be related to study medication. There were three AEs reported in the no Tx group: toothache in a tooth adjacent to a study tooth, ulcerative stomatitis, and melena. They were not related to study medication. An SRP patient reported an event of myalgia, and SRP patient reported an event of a gramulomatous lesion, and an MPTS patient reported an event of toothache in a non-study tooth, and SRP + MPTS patient reported an event of rhinitis, and an MPTS patient reported an event of headache. Regarding the gingival margin, the SRP + MPTS group tended to display the! least redness and swelling at 14 days and one month. The same trend was obsetved at six months, but to a lesser degree There was no significant difference in intensity and extent of tooth staining at any assessment point among the treatment groups. There -were no abnormal clinical chemistry or haemnatological results observed in the study for any of the groups. July 2015 Page 124 Scaling and root planing - Updated literature Citation: Author, Year Country Special population? (e.g. smokers) and other data regarding inclusion exclusion criteria for patients and teeth Severity of disease (e.g. refractory, mild, moderate) Test subject age (mean, median, range as described) Zhou, 2014 china patients with COPD. Exclude: asthma, pero tx in last 6 months chronic PD 63.9 +/- 9.44 July 2015 Control subject age (mean, median, range as described) Control Scaling group: 65.3 +/- 7.54. No 1. Supragingival Scaling; tx group: 68.0 +/2. No tx control 7.64 Control Dose/ duration/ frequency/ timing if applicable Test (typically adjunct) n/a SRP Test (typically adjunct) Was standard Trial design Dose/ duration/ frequency/ timing counseling (split mouth / Duration of including simultaneous/ before/ mentioned as part of parallel group study after control treatment? / cross over) timing not stated yes parallel 2 years Adverse events reported not reported Page 125 Scaling and root planing – Outcomes data Citation: Author, Year Outcome measure Time period for data presented in this abstraction Other time No. Sites treated per periods for mouth / No. sites averaged which data are per tooth available Test sample size Baseline Test mean Baseline Test SD or SE (list value) Baseline Test sample size at end of test period Test mean at end of test period Test SD or SE (list value) at end of test period SD or SE? Mean gain TEST (Baselinefinal) Mean SD (or SE) gain, TEST Control sample size Baseline Control mean Baseline Control SD Control sample or SE (list size at end on value) test period Baseline Control mean at end of test period Control SD or SE (list value) at end of test period SD or SE? Mean gain CONTROL (baseline-final) Mean SD (or SE) gain, CONTROL Caries data 8 NR NR 8 NR NR SD 0.7 0.3 8 NR NR 8 NR NR SD -0.3 0.3 NR Group 1: 45 Group 2: 45 Group 1: 3.57 Group 2: 2.95 Group 1: 1.31 Group 2: 1.21 Group 1: 42 Group 2: 43 Group 1: 3.20 Group 2: 2.55 Group 1: 1.23 Group 2: 1.16 SD Not reported Not reported Group 3; 44 3.37 1.24 Group 3; 41 3.41 1.23 SD Not reported Not reported Not reported Not reported Not stated 0.58 0.14 12 or 13 (not stated) 11.04 1 10 Not reported Not reported Not stated 0.08 0.16 Berglundh, 1998 PAL, overall (Groups 3 and 4) 12 months 2 months Chen 2012 CAL 6 months 1.5, 3 months 6 sites per tooth, full mouth assessment 1, 3, 6 months The "experimental sites" were two sites in each patient that met the entrance criteria of probing depths >7 mm and detectable levels of the target microbial species. The "study quadrant" was the quadrant in each patient that contained the experimental sites / CAL at 6 sites per tooth / study sites in the study quadrant were averaged per subject and subjects were averaged to create group means 12 or 13 (not stated) 10.77 1.22 6 Presented in graphic form with exact change difficult to tell. Text reports that at 6 months, there was a significant difference between the SRP group and the NoTx group (P <0.05, Duncan's multiple range test). Unclear. It appears that all teeth in each quadrant were analyzed. One quad 20 11.2 1.7 not reported 10.8 1.6 Assume SD from value and text CHANGE IN RAL LEVELS: Group 2 DHY moderate pockets:0.5m m. not reported 20 11.5 2.9 not reported 11.4 2.6 Assume SD from value and text CHANGE IN RAL LEVELS Group 4 moderate pockets: 0.2mm not reported not reported Unclear. It appears that all teeth in each quadrant were analyzed. One quad 20 13.6 2.2 not reported 12.5 2.4 Assume SD from value and text CHANGE IN RAL LEVELS: Group 2 DHY deep pockets: 1.2mm. not reported 20 12.9 1.4 not reported 12.4 2.5 Assume SD from value and text CHANGE IN RAL LEVELS Group 4 deep pockets:0.2mm not reported not reported se SRP mean difference = 1.4 SRP SE = 0.3 se SRP control difference = 0.4 SRP control SE = 0.4 Jones 1994 Q1 only Note: data read from figure; only max whisker value plotted in figure Mean change from baseline, CAL, mm 6 months RAL (Moderate pockets 3-6mm); estimated from figures 5 and 6 6 months Kahl, 2007 Also reporting Proportion of sites demonstrating >=2 mm PAL gain or loss at the12-month reexamination. No notes NA, severe Unit of analysis was the subject. Duncan's multiple caries range test was used to make comparisons were not between group means as well as group mean included changes from baseline. A value of <0.05 was in the considered statistically significant. The chisquare study. test was used to analyze data reflecting the No data percentage of sites showing change presented . Has data on use of Vector ultrasonic irrigated with hydroxylapatite particles (group 1). The power calculation revealed that when the sample size is 9, there is 80% power to detect a difference in means of 0.40 3 mo RAL (6-8mm); estimated from figures 5 and 6 Lindhe 1983 The examination included all teeth and 4 sites of each tooth; mesial, buccal, distal and lingual. For each variable, individual mean values were calculated. Statstical analysis notes No mention of tooth loss 102/4 CAL 1/2 mouth 6 months 50 weeks 10, 20 30 50 weeks 6 months 1 week, 1 month, 3 months 1/2 mouth 7 7.4 0.5 7 nr nr Not reported Not reported Not reported 7 7.9 0.6 7 nr nr Has data on use of Vector ultrasonic irrigated with hydroxylapatite particles (group 1). The power calculation revealed that when the sample size is 9, there is 80% power to detect a difference in means of 0.40 no t-test on difference, anova Not reported Comparisons by individual tooth utilized ANOVA, Kruskal-Wallis nonparametric ANOVA, and chisquare tests; SD or SE not defined; figure data different from text data Not reported Not reported Experimental unit was the subject in all statistical analyses. These data were adjusted by Duncan's multiple range test for multiple comparisons. Within each treatment group, data from baseline and 24 weeks were compared and analyzed by Student t-test. The data of the with-scaling and without-scaling were also compared and analyzed by Student t-test. Statistical significance was accepted at probability level of ≤0.05 for all tests. Mod: ± 1.46 Deep: ±1.04 NA 1.0 Neill 1997 (SRP vs no tx) DATA IN FIGURE AND TEXT INCONSISTENT Ng, 1998 CAL gain, mm CAL 24 weeks 3 weeks, 6 weeks, 12 weeks, 24 weeks 6 months Baseline, 3 and 6 months 10 pts, 186 total teeth: 91 laser, 49 SRP and 46 NT 10 people; 49 teeth Not reported Not reported 10 people; 49 teeth 8 (32 people divided among 4 test groups; each split between SRP and no treatment) CAL:9.0 CAL:1.9 8 CAL:9.9 CAL:0.8 SD Moderate: 7.61 Deep: 9.42 Mod: ± 0.94 Deep: ±1.92 13 Not reported Not reported SD 744 total sites: 364 laser, 196 SRP and 184 NT 1 site per treatment combination (many arms in trial) "All teeth were matched accoring to tooth type in both groups Note: different from figure, which plots approximately 1.3 mm 1.7 10 people; 46 teeth Not reported Not reported 10 people; 46 teeth Not reported Not reported 8 CAL:9.5 CAL:2.3 Moderate: 1.21mm Deep: 2.41 12 Mod: ± 0.98 Deep: ±0.92 Moderate: 7.72 mm Mod: ± 1.23 Not reported Not reported Not reported 8 CAL:10.4 CAL:0.5 SD 12 Not reported Not reported SD Estimated 1.3 Not given; from other Figure indicates data reported 0.5 in paper Not reported RAL (Relative Attachment Level) at baseline; RAL gain at endpoint "moderate" and "deep" pockets undefined Ribeiro (DEL PELOSO), 2008 An individual occlusal stent was fabricated of selfcuring clear resin to create fixed landmarks and to standardize the location and angulation of periodontal probes. RAL was measured from the stent to the bottom of periodontal pocket. At least 8 teeth with a PPD of ≥5 mm and BOP. At least 2 of the 8 qualifying teeth must have PPD≥7 mm, and the pockets of a further two teeth must have PPD≥6 mm; minimum of 20 teeth in both jaws (wisdom teeth excluded) 13 108 sites in the test group 104 sites in the control group Deep: 9.16 Deep: ±1.91 Moderate: 1.52mm Deep: 2.40 mm Power calculation to determine 1.0 difference in CAL; Friedman test used to detect intra- group differences in RAL, and the Mann–Whitney test was used to detect intergroup differences at each time interval. The Wilcoxon test was used to compare moderate and deep pockets in each group. There was no difference between groups regarding the proportions of sites presenting a RAL gain of >2mm at any experimental time. Operator training; examiner calibration for intra examiner reliability Site = unit of analysis, ReML method for fitting mixed model, full factorial for Er:YAG laser and SRP, post-hoc Tukey-Kramer honestly significant difference test Rotundo 2010 (SRP vs no tx) CAL 6 months 1 week, 3 and 6 months 27 pts, all teeth with at least 1 site with PD ≥ 4 mm; 6 sites/tooth Not reported 27 people; 422 sites 6.1 1.6 26 people; 399 sites 5.6 2 (based on value, assumed SD) Not reported Not reported 27 people; 387 sites 6.1 1.5 26 people; 365 sites Not reported 5.9 2.2 Not reported Not reported (based on value, assumed SD) Not reported The statistical analysis was intention to treat. In particular, if a patient showed up at the 3-month recall visit and not at the 6-month re-evaluation, the clinical data were imputed to the 6-month analysis The sample size was calculated using a=0.05 and the power (1- b)=80%. For the variability (s=SD), the value of 0.6mm (Sculean et al. 2004) was used considering clinical attachment level (CAL) gain as a variable outcome. The minimum clinically significant value (d) considered was 0.5mm Change in CAL (baseline given), mm 12 (10 included in this mean) 11.11 not given 12 0.54 0.18 SE 0.54 0.18 13 11.28 not given 13 0.24 0.25 SE 0.24 0.25 N/A No notes 6 months 12 (10 included in this mean) 11.11 not given 9 0.6 0.28 SE 0.6 0.28 13 11.28 not given 11 0.28 0.19 SE 0.28 0.19 N/A No notes 6 months 12 (10 included in this mean) 11.11 not given 10 0.45 0.2 SE 0.45 0.2 13 11.28 not given 12 0.23 0.3 SE 0.23 0.3 N/A No notes 6 months ALL TREATED SITES Van Dyke 2002 Months 1 and 3 Change in CAL (baseline given), mm six sites on two teeth selected for the study. The two teeth were selected based upon the presence in at least one of the sites (in each tooth) of A PD 6mm and GCF levels of PGE 2 > 66.2ng/ml. Although treatment was administ’d only at sites ≥5mm on the study teeth, clinical indices were monitored at all six sites. >=5 but <6 mm SITES Change in CAL (baseline given), mm >=6 mm SITES July 2015 Page 126 Scaling and root planing - Updated literature Citation: Author, Year Outcome measure Zhou, 2014 CAL July 2015 Time period for No. Sites treated data presented in per mouth / No. this abstraction (as sites averaged per close to 9 months tooth as possible) 1 year not stated Test sample size Baseline Test mean Baseline 20 4.58 Test Test SD or Mean Mean SD Test SD or SE sample Test mean at SE (list difference (or SE) (list value) size at end end of test value) at SD or SE? TEST (final- difference, Baseline of test period end of test baseline) TEST period period 1.72 20 4.02 1.27 SD NOT REPORTED NOT REPORTED Control sample size Baseline Control mean Baseline Control SD or SE (list value) Baseline 20 Scale: 4.33 NoTx: 5.27 Scale: 1.45 NoTx 1.69 Control Control SD Control sample or SE (list mean at end size at end value) at SD or SE? of test on test end of test period period period Mean difference CONTROL (finalbaseline) Mean SD (or SE) Caries data difference, CONTROL Scaling group: Scaling: 1.05 3.93 No treatment: No 1.59 treatment:5.49 NOT REPORTED NOT REPORTED 20 for scaling group, 19 for no treatment SD not reported Statstical analysis notes 2-sided hypothesis, signifcance set at <0.05 Page 127 Scaling and root planing – Review authors’ judgment of each risk of bias item as low, unclear, or high for each included study Selection bias Domain: Random sequence generation Allocation concealment Masking of participants Review author's judgment Support for judgment randomly created two different samples assigned sample to tx High computer generated low Citation: Author, Year Support for judgment Berglundh, 1998 Chen 2012 Jones 1994 Details not given Performance bias Review author's judgment Support for judgment Not described unclear Allocation was concealed from investigator low Masking of personnel Review author's judgment Support for judgment blind, placebo used Low not described, but not likely Unclear Were the groups treated the same except for the intervention? Review author's judgment Support for judgment no mention of masking or blinding of examiner or therapist high not described, but not likely Unclear Detection bias Attrition bias Reporting bias Masking of outcomes assessment Incomplete outcome data (Include percent lost to follow-up) ≤10% low; 11-20% unclear; >20% high Selective reporting Review author's judgment Support for judgment Review author's judgment "if indicated OHI" High no mention of examiner blinding High Groups 1 and 2 received OHI, group 3 (control) did not high not described Unclear less than 10% attrition low all data was reported low Low 39/51 completed (24% lost) from all arms; test group about 50% lost High No bias detected Low Support for judgment Review author's judgment Support for judgment Review author's judgment no evidence of clinical trial registration none lost Low Unclear PAL reported but not PD or other perio measures Unclear No details given Unclear Attemp made to mask Low Not described Unclear Yes Low Blinded at all points in time but sometimes SRP tx was obvious high not possible high not possible high Yes Low examiner was blinded Low No dropouts occurred low all data was reported low Kahl, 2007 Drawing of a lot low senior author, who was not involved in the treatment, knew the allocation Lindhe 1983 randomized but no method reported unclear not reported Unclear double blinded but no details Unclear double blinded but no details Unclear Yes Low double blinded but no details Unclear no info. Assumed no loss Unclear No bias detected Low Neill 1997 States random assignment but does not specify how Unclear States double blind but does not explain Unclear No details given but "double blind" Unclear No details given but "double blind" Unclear OHI stated as given to control but not mentioned if given to other groups Unclear No details given but "double blind" Unclear No loss to follow up; data details sparse Unclear All data reported Low Ng,1998 randomized but no details Unclear not discussed high Not possible to mask participants to SRP/no tx high Not possible to mask personnel to SRP/no tx high Yes Low Low none lost Low reported on primary&second ary outcomes Low Ribeiro (DEL PELOSO), 2008 Computer generated Low another person not involved in the study Low Not possible High Not possible High Yes Low Low 0 lost Low No bias detected Low Low Envelope not opened utnil phase II tx Low No Unclear Examiner was always blinded as to treatment Low Yes Low Examiner was always blinded as to treatment Low 24/26 completed to 6 months; 1 lost; 1 ITT only 3 month data Low All data reported Low Unclear Details not provided Unclear control subjects received irrigation low "evaluator blinded" low Yes Low Evaluator masked Low Up to 20% lost Unclear No bias detected Low Random assignement by quad, opaque sealed envelope sequentially Rotundo 2011 number, computer generated random permuted block Van Dyke 2002 No details given examiners all masked to treatment group assignment Outcome measurements by blinded examiner Scaling and root planing - Updated literature Selection bias Domain: Random sequence generation Citation: Author, Year Zhou, 2014 Support for judgment computer generated list July 2015 Performance bias Allocation concealment Review author's judgment Support for judgment low identification was concealed fromall individuals directly involved in the study until final examinations and data collection had been concluded Review author's judgment low Masking of participants Support for judgment not possible Review author's judgment high Masking of personnel Support for judgment not possible Review author's judgment high Were the groups treated the same except for the intervention? Support for judgment yes Detection bias Attrition bias Reporting bias Masking of outcomes assessment Incomplete outcome data (Include percent lost to follow-up) ≤10% low; 11-20% unclear; >20% high Selective reporting Review author's judgment Support for judgment Review author's judgment Support for judgment Review author's judgment Support for judgment Review author's judgment low examinations were conducted by two trained dentists who were blinded to the study design low 0 lost at 6 months, 1/60 lost at 1 year, 2/59 lost at 2 years low reported all data low Page 128 Systemic low-dose doxycycline + SRP - Study characteristics Citation: Author, Year Special population? (e.g. smokers) and other data regarding inclusion exclusion criteria for patients and teeth Country Severity of disease (e.g. refractory, mild, moderate) Control Test subject subject age age (mean, (mean, median, median, range as range as described) described) Control Control Dose/ duration/ frequency/ timing if applicable Test (typically adjunct) Test (typically adjunct) Was standard Trial design Dose/ duration/ counseling (split mouth frequency/ timing mentioned as part / parallel including of control group / simultaneous/ treatment? cross over) before/ after Duration of study Adverse events reported Other time periods for which data are available OHI, written one or two sessions of instructions, and SRP with a maximum demonstration of Bass of seven days method, use of between at the interdental brushes and Parallel baseline visit, and floss were provided (multicenter) doxycycline hyclate each subject, both (20 mg, twice/day) control and test started at the baseline groups, at each visit for 3 months, treatment session. 12 months Not reported Baseline, 3, 6, 9 and 12 months Type 1 and Type 2 Diabetics Paper did not give % of smokers in study population. Al Mubarak 2010 Saudi Arabia Caton 2000 USA Deo 2010 India Emingil 2004 "Effectiveness ..." CITE TOGETHER Turkey WITH Emingil 2004 "The Effect of..." SAME CAL DATA A specific diagnosis was not one or two sessions of SRP with a stated. Inclusion criteria: diagnosis of maximum of seven days between at type I or II diabetes ≥ 1 yr; the baseline visit and placebo tablets diabetes under control by insulin Based on age of population twice/day, started at the baseline 48 +/- 5 years 51 +/- 6 years Twice/day, started at the or oral hypoglycemic agent or and the inclusion criteria visit, for 3 months (Group 2) (Group 1) baseline visit for 3 months both; constant type and dose of stipulating 5 mm to 8 mm diabetic medication administered PPD, a diagnosis of moderate full mouth supra and subgingival to severe chronic for previous 6 mo; good physical debridement using ultrasonic and periodontitis seems condition ; no serious medical hand instrumentation appropriate. issues or transmittable diseases; no cardiac condition requiring antibiotic prophylaxis; ≥ 18 remaining natural teeth with a minimum of 6 sites in at least 2 BID (once in am, once in periodontitis (at least 2 tooth pm); 1 hour before meals sites within each of 2 74% were past smokers, 40% qualifying quadrants with 45: range 30- 47 range: 31SRP performed until the SRP with Placebo were current smokers PD and CAL between 5 and 72 74 tooth and root surfaces 9mm, inclusive, which bled were free from deposits on probing as determined by visual or tactile examination SRP+placebo / scaling and root planing (SRP), using manual (standard Gracey currettes) and powered instruments (EMS±mini placebo capsule b.i.d. for 37.1 ± 3.96 years Pts with diabetes Chronic periodontitis Piezon) within 24 hours by the same the treatment period of six examiner. Root surfaces were months instrumented until they became free of deposits as determined by visual or tactile examination. four to six sessions by the same examiner. Tooth and root surfaces were instrumented under local Chronic periodontitis anesthesia up to 1 hour allowed per quadrant until No Study patients had at least they were free of all 14 natural teeth, at least deposits as determined None of the subjects were heavy SRP+ placebo capsules eight pockets with ≥5 mm by users of alcohol or tobacco. 44.90 ± 5.06 47.80 ± 8.40 probing depth (PD) and ≥4 visual or tactile They were smoking less than five 37-53 39-61 placebo capsules mm clinical attachment level examination. cigarettes per day. NOTE - 3 in b.i.d. for 3 months (CAL), and radiographic LDD and 5 in placebo group evidence of moderate to Patients were also noted as "current smokers" advanced chronic encouraged to continue periodontitis good oral hygiene and given regular maintenance therapy (i.e., removal of any supragingival plaque and calculus) at every recall No Emingil 2008 Turkey Smoking status was classified as nonsmoker or current smoker. Smokers in both treatment groups were smoking less than five cigarettes per day for >5 years. Subjects who had never smoked or who had stopped smoking ≥5 years ago were considered non-smokers. 4 in SDD and 6 in placebo No Emingil 2011 STUDY RELATED TO EMINGIL 2006 Turkey July 2015 All subjects were either non-smokers or smoked <5 cigarettes per day (11 in the SDD and 10 in the placebo group) and stayed as such during the course of the study. SRP and LDD (doxycycline hyclate) SRP with doxycycline 20mg BID 1 hour before meals; for 9 months Not reported parallel group 9 months table of adverse events provided; 3 dropouts due to adverse events: 1 test and 2 3, 6, 12 months from placebo Systemic SDD doxy+SRP doxycycline hyclate 20 mg twice a day (b.i.d) for 6 months No Parallel group 6 months During the course of the study, wound healing was uneventful. No patient showed adverse reaction to SDD therapy. None of the selected patients dropped out before the termination of the study. Patients received oral hygiene instruction at each session, including toothbrushing Parallel group and the use of interdental flossing or interdental brushing as appropriate. 12 months Collected throughout the study; Treatment with LDD was well tolerated and none of the patients reported any adverse effects 3, 6, 12 months from the use of either LDD or placebo capsules. The treatment included SRP that was carried out in four to six sessions by Chronic periodontitis the same examiner (GE). Oral hygiene Tooth and root surfaces instruction was given The subject All subjects had ≥14 natural 47.83 +/– 7.9 were instrumented under SRP+subto the subjects at 44.50 +/– 5.2 was given motivation to reinforce the teeth, at least eight pockets local anesthesia (up to 1 antimicrobial dose 20 mg, twice a day for each session on oral hygiene as well as the regular Parallel group with probing depth (PD) ≥5 range: 39 to hour per quadrant) until doxycycline 3 months toothbrushing and the range:37 to 53 maintenance therapy, i.e., removal of mm and clinical attachment 61 they were free of all (SDD) use of flossing or any supragingival plaque and level (CAL) ≥4 mm, and deposits as determined interdental brushing calculus took place at every visit radiographic evidence of by visual or tactile as appropriate during the 6-month period. moderate to advanced examination / placebo chronic periodontitis capsules twice a day for 3 months 6 months Treatment with SDD was tolerated well, and none of the subjects complained of any adverse effects from the use of SDD or placebo capsules. 3 months 12 months Adverse event information was collected at each visit. Treatment with SDD was welltolerated and none of the patients reported any adverse effects from the use of either SDD or placebo capsules. 3, 6, 9, 12 months SRP+Low-Dose Doxycycline (LDD) Therapy: 20 mg capsule; taken b.i.d. for 3 months; 1 hr before eating morning and evening. Adjunctive to SRP (20 mg, b.i.d.) for 3 months None SRP+placebo Chronic periodontitis. All patients were diagnosed with moderateto-severe chronic periodontitis according to clinical and radiographic findings and periodontal history Study patients had at least 14 natural teeth, at least eight pockets with ≥5 mm probing depth (PD) and ≥4 mm clinical attachment level (CAL), and radiographic evidence of moderate to advanced chronic periodontitis scaling and root planing (SRP), which was carried out in four to six Patients received oral sessions by another hygiene instruction at oral hygiene was reinforced and clinician. Dental surfaces SRP+ SDD (20 mg each session, regular maintenance therapy (i.e., were instrumented under subantimicrobialper capsule; 2 x 1) including removal of any supragingival plaque Parallel group local anesthesia with <1 dose doxycycline was given at baseline toothbrushing and the and calculus) was performed at hour allowed per (SDD) for 3 months. use of interdental every recall visit quadrant until they were cleaning as free of all deposits as appropriate. determined by visual or tactile examination. SRP+placebo 46.1 +/– 6.4 years 34 to 59 years 47.7 +/– 7.6 years 35 to 61 years Page 129 Systemic low-dose doxycycline - Study characteristics (continued) Citation: Author, Year Gürken et al. Int J Dent Hyg 2008;6:84-92. Haffajee 2007 Special population? (e.g. smokers) and other data regarding inclusion exclusion criteria for patients and teeth Country Control Test subject subject age age (mean, (mean, median, median, range as range as described) described) 35 males and female pts. Inclusion criteria included systemically healthy, at least 3 natural teeth in each quadrant Diagnosed as having and a total of 14 teeth. No pt. severe generalized chronic had a drug allergy, used periodontitis defined as: > NSAIDS, had taken an antibiotic 46.38+-7.63 46.77+-6.95 30% of sites with ≥ 5 mm or received perio therapy in (range: 34-59) (range: 35-59) CAL and having at least 2 previous 3 months. Women sites with PD ≥ 6 mm in could not be pregnant, actively each quad with BOP. breast feeding or use oral contraceptives. 4 and 5 smokers in SDD and placebo groups, respectively. Turkey U.S. Severity of disease (e.g. refractory, mild, moderate) No at least eight sites with PD>4 mm / no perio tx within previous 3 months / not pregnant or nursing / no systemic conditions requiring antibiotics Moderate chronic periodontitis 47 +/- 11 43 +/- 15 Control SRP+ Placebo SRP at baseine and then at 3 maintenance SRP visits post baseline Control Dose/ duration/ frequency/ timing if applicable Test (typically adjunct) SDD group received a 20 mg Placebo capsules taken capsule; taken b.i.d. 1 hr before morning b.i.d. 1 hr before and evening meals for 3 morning and months evening meals for 3 months plus SRP SRP was performed a quadrant at a time under local anaesthesia at approximately weekly intervals SRP+Systemic Periostat (SDD doxycycline) Test (typically adjunct) Was standard Trial design Dose/ duration/ counseling (split mouth frequency/ timing mentioned as part / parallel including of control group / simultaneous/ treatment? cross over) before/ after SDD 20 mg.; b.i.d. (12 hr intervals) for three months adjunctive to SRP 20 mg doxycycline (SDD, Periostat) b.i.d. for 12 weeks / started at first SRP visit Authors did not report giving OHI or other Parallel group instructions No Parallel group Duration of study Adverse events reported Other time periods for which data are available 12 months The authors reported no adverse events. Baseline, 3, 6, 9 and 12 months 12 months Two subjects in the SDD group reported adverse events including dizziness /tachycardia with the administration of local anaesthetic for SRP and a possible interaction between the study medication and OTC vitamins 3 and 6 months and calcium supplements. The subject stopped taking the latter for the duration of the SDD administration. No adverse events were reported in the SRP-only group Institutionalized geriatric patients aged 65 or older / equal exsmokers in each group (not excluded) baseline clinical attachment levels (CAL) 5–9 mm, probing depths (PD) 4–9 mm and bleeding on probing Mohammad 2005 USA Needleman 2007 UK Smokers; at least 16 teeth U.S. 210 males & females comprised the study population. Inclusion criteria were: Evidence of periodontitis (i.e., CAL and PD between 5-9 mm with BOP in two sites in each of two quads). Exclusion criteria were: pregnancy, lactation, serious chronic medical condition (e.g., diabetes, kidney or liver disease), acute syst infection, previous prophy or perio Tx within 90 days of baseline, requirement for antibiotic prophylaxis prior to dental Tx, use of non-TCH antibiotic with 3 months of baseline, hypersensitivity to TCH, or the need for long-term (> 2 wks) daily antacids containing aluminum, calcium or magnesium and participation in a perio clinical trial within 12 months of baseline. 26 (25.5%) subjects in control group were smokers. 41 (38.3%) subjects in SDD group were smokers. Preshaw et al. JP 2004 moderate-severe chronic periodontitis Excluded if:chronic use of NSAIDs, steroids or antibiotics, although use of subanalgesic doses of aspirin for anti-platelet aggregation was permitted / no serious medical condition / no diabetes / no perio therapy within the last 6 months chronic periodontitis Based on inclusion criteria of CAL and PD between 5 and 9 mm with BOP, patients presented with moderate and severe periodontitis. Radiographic exam was used to establish Dx of chronic periodontitis and severity of disease. 81 (range 72- 83 (range 7793) 90) Mean=41.7 mean birth year 1960.3 SD 7.6 Mean=44.2 mean birth year 1957.8 SD 6.1 SRP + placebo SRP at baseline and 20 mg doxycycline 2x daily for 3 months post baseline SRP using local anesthesia and manual and ultrasonic Mean age of Mean age of instrumentation. Following SRP, 48 yrs with a 48 yrs with a subjects weere randomized into range of range of 34control and test groups. Authors did 35-75 yrs. 75 yrs. not state who did SRP, i.e., RDH or DDS. At month 9, all subjects received a dental prophy and were exited from study. performed until the crown and root surfaces were visually and/or tactilely free of all deposits / placebo twice daily for SRP + Systemic the treatment SDD doxycycline period of the study (9 months), commencing at baseline; one pill in morning and one in evening 20 mg doxycycline 2x daily for 3 months post baseline Control group took a placebo pill, b.i.d., 1 hr prior to meals with a 12 hr interval. Placebo and test SDD were identical in appearance and dispensed in 3-month quantities for 9 months SDD; 20 mg doxycycline twice daily commencing at baseline; one pill in morning and one in evening 20 mg SRP with 20mg doxycycline 2x doxycycline daily for 3 months (Periostat) BID for3 post baseline months post baseline SRP + SDD (doxycycline hyclate) SDD, 20 mg, b.i.d., 1 hr prior to morning and evening meal with a 12 hr interval. SDD was dispensed in 3 month quantitie for a total of 9 months. At every appointment oral hygiene procedures were reinforced. Following parallel group the month 9 visit, a further dental prophylaxis was provided. 9 months Not reported 3 and 6 months all subjects received smoking cessation counseling prior to study; all subjects received OHI after randomization 6 months 5 subjects, all in placebo group; minor issues such as pulpal or periodontal problems 1 and 3 months, but not reported in paper Not Reported parallel group Double-blind, radomized, placebocontrolled, multicenter, parallel group. Examiners were calibrated. 9 months contacted monthly by telephone for the recording of adverse events (AEs) No (smokers included) Preshaw 2008 US and UK untreated periodontitis manifested by four periodontal sites in each of two quadrants, i.e., eight qualifying periodontal sites, with aminimum of two affected teeth per quadrant, with all eight qualifying sites demonstrating probing depth (PD) ≥5 mm, attachment loss≥ 5 mm, and bleeding on probing (BOP) score ≥1, with at least two sites having bleeding scores≥2 / not pregnant or nursing / no serious medical conditions or systemic infections / no dental prophy within the last 30 days / no non-tetracycline antibiotics within 6 weeks of baseline; no use of tetracycline antibiotics within 3 months of baseline; July 2015 The % of subjects who experienced Txemergent adverse events (i.e., all causes) was similar for control (61%) and SDD (59%) subjects. Most frequent adverse events were: infection, headache, pain and influenza. There were no statistical differences between placebo and SDD groups regarding GI tract genito-urinary Baseline, 3, 6 tract, photosensitivity, hypersensitivity, and 9 months enterocolitis, fever and lymphadenopathies. One control subject had an MI and one had severe asthma. One SDD subject had chest tightening, was hospitalized, but successfully completed study. None of the serios adverse events were considered related to study product. Periodontal disease (unspecified) 48.5 ± 11.4 24 to 81 49.9 ± 11.0 23 to 82 SRP+placebo SRP completed within a maximum of 24 hours. SRP was performed by supra- and subgingival use of ultrasonic instruments followed by hand instruments for removal of plaque and calculus deposits until tooth surfaces were smooth and calculus could not be detected, with a time limit of 1 hour per quadrant. Ultrasonic and universal or areaspecific curets were used, as was local anesthesia, based on operator preference In the SDD-40 group, 88 subjects (66.2%) reported a total of 217 AEs; 94 subjects (70.7%) reported a total of 229 AEs in the placebo group Systemic SDD doxy+SRP Oracea; 40 mg modified release dose, 1x/day for 9 months Not mentioned Parallel group 9 months In the SDD-40 3 and 6 months group, the most frequently reported AEs were headache (13 subjects; 9.8%) and influenza and nasopharyngitis (each Note that % seven subjects; 5.3%).In the placebo sites with CAL group, the most frequently reported AEs gains greater were sensitivity of teeth (13 subjects; than 2 or 3 mm 9.8%) and headache and nasopharyngitis is provided (each 10 subjects; 7.5%). AEs were ranked as mild, moderate, or severe in intensity, with the most common being mild or moderate. Severe AEs were recorded for nine (6.8%) subjects in the SDD-40 group and 18 (13.5%) subjects in the placebo group. No AE was considered to be probably related to the study medication in either group Page 130 Systemic low-dose doxycycline - Outcomes data July 2015 Page 131 Systemic low-dose doxycycline - Outcomes data (continued) July 2015 Page 132 Systemic low-dose doxycycline - Review authors’ judgment of each risk of bias item as low, unclear, or high for each included study Selection bias Domain: Random sequence generation Review author's judgment Citation: Author, Year Support for judgment Mubarak, 2010 Subjects at each center were allocated numbers which were then used to randomize the four different groups (2 control and 2 test groups). This was achieved by distributing the subject number among the four study groups by sequentially allocating them to one of four alphabetical codes relating to a test group, i.e., A for Group 1, B for Group 2, etc. low Caton 2000 computer generated list low Deo 2010 Emingil 2008 Emingil 2011 Allocation concealment Masking of participants Review author's judgment Support for judgment Review author's judgment Reporting bias Masking of outcomes assessment Selective reporting Support for judgment Review author's judgment Review author's judgment Support for judgment Review author's judgment Did not include those who took other medications, that did not show for appointments etc. Low No bias detected in design or reporting of data. Low Unclear 10% low No bias detected Low Unclear No losses Low No bias detected Low Support for judgment Not Reported Unclear Subjects were also blinded to the prescribed medication (doxycycline hyclate, 20 mg or placebo). Low SRP provided by hygienists who were blinded to assigned treatment Low Yes low Examiners were blinded low No details given Unclear double-masked low double-masked low yes low Masking not mentioned Low Masking not mentioned, unmarked placebo capsules Unclear Yes Low Masking not mentioned Support for judgment 23 of 369 subjects did not complete the 12 month study (6.2%) No details given Unclear No details given Unclear Coin flip Low Study medications were identical in appearance. Low Double blind with placebo control group Low Double blind; Examiner recording data was unaware of treatment. Low No differences noted Low Examiner masked Low 20/30 completed 12 months High No bias detected Low Coin flip Low Study medications were identical in appearance Low Yes, and identical capsules Low double blinded, but no details Unclear No differences noted Low Examiner blinded Low Although 24/30 completed, power calc indicates this is adequate size Unclear No bias detected Low Low placebo capsules identical in appearance but containing inactive filler; All patients were represented with a code; coded bottles Low Double-masked Low Not specified Unclear No differences noted Low Clinical examiner masked Low 23/32 and 23/33 (70-72%) completed High No bias detected Low Low Not stated who was responsible for coin toss. unclear Double blind with placebo control group Low Double blind Low Yes Low Examiner recording data was unaware of treatment Low 11 lost (25%) High No bias detected; reported primary and secondary outcomes Low Unclear No - single blinded High No-single blinded High Yes Low Masked Low 67/98 subjects had complete data for all monitoring visits; ITT used with LOCF; if only baseline data, subject excluded High AL at 12 months for pockets with baseline PD>6 mm primary outcome measure Low Unclear Identical pills Low Nurses masked to allocation Low Randomization after SRP Low Unclear if masked Unclear No losses Low No bias detected Low Low blinded, randomization not broken until after data analysis; concealed containers Low all patients given SRP and either doxycycline or placebo Low blinded until after data analysis Low 2/18 in placebo, 2/16 in control did not complete study (none due to adverse events). Intention to treat analysis used. Low reported on primary&secondary outcomes Low Low "Double-blind"; assume this was the SRP procedure prior to adjunct Low Low Masking not mentioned Unclear 34/102 in control group, 18/107 in intervention group lost High No bias detected; reported primary and secondary outcomes Low Coin flip Gürkan et al. Int J Dent Pt assigned by coin toss Hyg 2008;6:84-92. Haffajee 2007 Random number table Low Assignment by clinic cocoordinator Mohammad 2005 computer assigned randomisation numbers Low No details given Needleman, 2007 randomized by comptuer Low allocation concealed until after data analysis; central randomization Low blinded, randomization not broken until after data analysis; concealed containers Preshaw et al. JP 2004 randomized but no details Uncelar Did not Report Unclear Placebo and SDD were identical in appearance and all received SRP July 2015 Support for judgment Were the groups treated the same except for the intervention? Attrition bias Incomplete outcome data (Include percent lost to follow-up) ≤10% low; 11-20% unclear; >20% high Review author's judgment Support for judgment Review author's judgment Masking of personnel Detection bias Indistinguishable placebo capsules Emingil 2004 "Effectiveness..." CITE TOGETHER WITH Emingil 2004 "The Effect of..." SAME CAL DATA Performance bias all patients given SRP and either doxycycline or placebo Page 133 Systemic antimicrobials: amoxicillin-metronidazole + SRP - Study characteristics Citation: Author, Year Country Berglundh, 1998 Sweden Cionca 2009 Flemmig 1998 Goodson, 2012 Special population? Severity of disease (e.g. Test subject age (e.g. smokers) and other data refractory, mild, (mean, median, regarding inclusion - exclusion moderate) range as described) criteria for patients and teeth no mention of cigarettes or any health condition Advanced periodontal disease 35-58 years adult chronic periodontitis with the presence of at least four teeth with a No. Smoking history was recorded, probing depth (PD) >4 Switzerland but smoking was not an exclusion mm, clinical attachment criterion. loss (AL) ≥2 mm, and radiographic evidence of bone loss. Germany USA and Sweden untreated periodontitis-4 pockets > 6 mm : moderate no mention of smoking Any systemic condition such as AIDS or diabetes excluded; Smokers included; no information on past smokers Control subject age (mean, median, range as described) Moderate to severe periodontitis; 4 teeth with pockets > 5 mm and 8 teeth with CAL > 3 mm 50.6 +/– 8.6 50.5 +/– 13.6 48.9 54.4 46 ± 2.0 47 ± 2.6 Control Control Dose/ duration/ frequency/ timing if applicable Test (typically adjunct) SRP+placebo local anesthesia 3-5 sessions unspecified operator scaling + AMX MET Mombelli 2005 Switzerland Ribeiro, 2009 Brazil Untreated perio; presence of inter-proximal periodontal lesions in each of tw o contra-lateral quadrants in the region including the canine, premolars, and the mesial aspect of the first molar, and presence of Porphyromonas gingivalis in a pooled subgingival plaque sample from this region / no systemic illness / not pregnant or lactating / systemic antibiotics taken w ithin the previous 2 months / no subgingival scaling in the last year Exclusion criteria were as follows: periapical alterations on qualifying teeth, medical disorders that require prophylactic antibiotic coverage or that could influence the response to treatment, scaling and root planing in the preceding 6 months, consumption of drugs known to affect periodontal status (antibiotic, anti-inflammatory, anticonvulsant, immunosuppressant, or calcium channel blocker) within the past 6 months, orthodontic therapy, allergy to penicillin and/or metronidazole, pregnancy and smoking Moderate to advanced chronic periodontitis Severe Chronic Periodontitis Inclusion age range criteria: Age 25–65 years 46 (range:34-55) 46.2 (range 30-66) AMX 375 mg tid metronidazole 250 mg tid for 14 days The operator treated at least half of the periodontally diseased teeth w ith thorough scaling and root planing to the depth of the pocket under local anesthesia. Ultrasonic instruments† w ere used first, follow ed by Gracey curets. SRP+Systemic The pockets w ere irrigated w ith a 0.1% aqueous SRP + metronidazole and solution of chlorhexidine, and the subjects w ere chlorhexidine + amoxicillin instructed to rinse the m outh w ith 0.2% placebo combined + chlorhexidine tw ice daily for the next 10 days. The chlorhexidine operator treated the remainder of the dentition in the same w ayw ithin 48 hours.At the end of the final treatment visit, the subjects received the medications and w ere instructed on how to take them. scaling SRP and CHX rinsing local anestehsia 2 hours per quadrant by students - non-periodontist instructor if student failed under local anaesthesia in 4 weekly visits given by therapist with unspecified qualifications / 0.12% CHX rinse 2xday scaling + AMX MET SRP + AMX MET(+ CHX rinse) No (did not exclude smokers) / Four subjects treated w ithout antibiotics, and tw o treated w ith antibiotics w ere smokers. With regards to pocket depth reduction and CAL gain there seemed to be a tendency of better clinical responses in non-smokers, but small numbers precluded a statistical evaluation. Test (typically adjunct) Trial design (split Was standard counseling Dose/ duration/ frequency/ mouth / parallel mentioned as part of timing including simultaneous/ group / cross control treatment? before/ after over) No mention of anesthesia at most 45 minuntes of scaling and no specification of operator scaling + AMX MET Parallel group for these data (Factorial) Adverse events reported 12 months NR metronidazole, 500 mg, and amoxicillin, 375 mg, to be taken three times per day for 7 days. Addition of subgingival irrigation with 0.1% and rinsing bid with 0.2% for 10 days Instruction on proper oral hygiene given to all pts Parallel group 6 months Recorded at 1 w eek, 3 and 6 months but only reported at 1 w eek during the active period of medication. The number of subjects presenting w ith stomach upset w as similar in both groups (n = 4 in the placebo group and n = 5 in the test group). How ever, the number of subjects complaining about gastrointestinal problems, notably diarrhea, w as higher in the test group (n = 6 versus n = 3). Cramps w ere noted in five subjects in the test group. Tw o teeth, in tw o subjects, w ere lost during the study, and tw o subjects show ed evidence of suppuration despite therapy; these events occurred in the placebo group. AMX 375 mg tid metronidazole 250 mg tid + 0.06% CHX for 8 days OHI at 3 month intervals parallel 12 months 4/18 on AB had GI problems Parallel group 24 months 1 dropped out due to nause vomiting and development of oral candidiasis (miconazole) 1 developed diabetes the patients were instructed in proper oral hygiene Parallel group 12 months Not reported yes parallel 6 months reported -4 patients had GI from test group and three from control group Each patient in the study was provided a powered toothbrush (3D; Oral B; Boston, MA) and a triclosan- containing toothpaste (Total®; Colgate; Piscataway, NJ, USA), and appropriate 500 mg AMX bid + 250 mg MET devices for approximal tid for 14 days tooth cleaning. Patients were instructed to perform the oral hygiene procedures twice daily.At each followup visit (3, 6, 12, 18 and 24 months) the patients’ oral hygiene standard was checked and reinforced when indicated. The two study teeth were treated as follows in a single, separate session: Thorough scaling and root planing to the depth of the periodontal defect, followed by pocket irrigation with saline solution, manual compression of gingival tissues for 5 min, and the placement of a retraction chord containing 10% potassium sulphate (Gingibraid 3a, Van R Dental Products, Oxnard, CA, USA). 250 mg metronidazole SRP + Placebo Next, an envelope containing the code for the Systemic AMXand 375 mg amoxicillin were medication + randomized supplementary treatment was MET + SRP + no administered together three times Placebo gel opened, assigning Emdogains to one, and Emdogain a day for 7 days. placebo to the other side. The retraction chords were removed after 2 min in place, and then the sites were rinsed with saline. PrefGel was applied in the pockets during 2 min (Blomlo¨f & Lindskog 1995, Blomlo¨f et al. 1996), followed by another saline irrigation / Two placebos were administered three times a day for 7 days. SRP oral hygiene instruction and one session of supragingival scaling. The oral hygiene instruction was, if indicated, reinforced during the course of the trial. Duration of study AMX 375 mg and 250 mg MET tid for 7 days Systemic antimicrobials: amoxicillin-metronidazole + SRP - Updated literature Citation: Author, Year Country Special population? (e.g. smokers) and other data regarding inclusion exclusion criteria for patients and teeth Severity of disease (e.g. refractory, mild, moderate) Test subject age (mean, median, range as described) Control subject age (mean, median, range as described) Control Control Dose/ duration/ frequency/ timing if applicable Test (typically adjunct) Miranda, 2014 Brazil pts with diabetes chronic perio; more than 30% of the sites with PD and clinical attachment level (CAL) ≥4 mm and a minimum of six teeth with at least one site with PD and CAL ≥5 mm and bleeding on probing (BoP) at baseline. 54.0 +/- 8.2 53.7 +/-8.0 Placebo and SRP 3 times day for 14 days SRP and MTZ and AMX July 2015 Test (typically adjunct) Was standard Trial design Dose/ duration/ frequency/ timing counseling (split mouth / Duration of including simultaneous/ before/ mentioned as part of parallel group study after control treatment? / cross over) 400 mg MTZ and 500 mg AMX three times a day for 14 days Yes parallel group 1 year Adverse events reported Diarrhea, headache, metallic taste, nausea/vomiting; However, no significant differences were observed between groups for the number of subjects reporting specific adverse effects Page 134 Systemic antimicrobials: amoxicillin-metronidazole + SRP - Outcomes data Citation: Author, Year Outcome measure Time period for data presented in this abstraction Other time periods for which data are available No. Sites treated per mouth / No. sites averaged per tooth Test sample size Baseline Test mean Baseline Test SD or SE (list value) Baseline Test sample size at end of test period Test mean at end of test period Test SD or SE (list value) at end of test period SD or SE? 8 NR NR 8 NR NR SD Mean gain TEST (Baseline-final) Mean SD (or SE) gain, TEST 0.8 0.4 Control sample Control mean size Baseline Baseline Control SD or SE (list value) Baseline Control sample Control mean at Control SD or SE size at end on end of test (list value) at end test period period of test period SD or SE? Mean gain CONTROL (baseline-final) Mean SD (or SE) gain, CONTROL 0.7 0.3 Caries data Statstical analysis notes 102/4 PAL, overall (Groups 2 and 4) Berglundh, 1998 12 months 2, 24 months PAL 4-5 mm pockets Cionca 2009 PAL >=6mm pockets CAL = PD +REC, where REC=positive if gingival margin located apical, negative if located coronal to the cemento-enamel junction Baseline, 3 months 6 months CAL gain, in mm [change in CAL from baseline} The examination included all teeth and 4 sites of each tooth; mesial, buccal, distal and lingual. For each variable, individual mean values were calculated. 25 Subjects in the Note: Number of test group had 26.9 pockets with PD>4 mm +/- 15.6 A total of 3,126 sites (six decreased from 30 to 3 sites with PD >4 per tooth) were clinically for placebo and 27 to mm and BOP. monitored at baseline and 0.5 for test at 3 and 6 months 9 months 137/6 PAL 4-6 mm 9 months 137/6 PAL >= 7mm 9 months 137/6 3,6,9 months NR, but 48 total were enrolled in beginning, and 38 completed the study NR, but 48 total were enrolled in beginning, and 38 completed the study NR, but 48 total were enrolled in beginning, and 38 completed the study NR NR 8 NR NR SD NR 0.6 0.3 0.7 0.3 1.8 0.5 1.3 0.4 6 sites of each tooth with a pocket >4 mm at baseline PAL <=3 mm Flemmig 1998 No mention of tooth loss 8 Not reported Not reported 23 Not reported Not reported SD 0.9 0.4 26 Subjects in the placebo group had 29.9 – 17.1 sites with PD >4 mm and BOP. NR NR 18 NR NR NA 0.7 (estimated from graph; not precise) NR NR, but 48 total were enrolled in beginning, and 38 completed the study NR NR 18 NR NR NA 0.85 (estimated from graph; not precise) NR NR NR 18 NR NR NA 1.3 (estimated from graph; not precise) NR 6.35 0.27 26 NR NR SE 1.53 0.16 8 2.1 One systemic AMX/Met and one placebo unavailable for 12month evaluation not reported not reported Not stated 1.7(estimated from graph) Not reported Not reported SD 1.62 8.15 (all pockets) 0.55 13 NR, but 48 total were enrolled in beginning, and 38 completed the study NR, but 48 total were enrolled in beginning, and 38 completed the study Also reporting Proportion of sites demonstrating >=2 mm PAL gain or loss at the12-month re-examination. Not reported Not reported 24 Not reported Not reported SD 0.9 0.4 Not reported The differences between patients in the treatment groups were determined at each time point using the Mann-Whitney U test; Backward stepwise logistic regression was used to study various relationships between results; comparisons statistics between groups not described NR NR 20 NR NR NA 0.8 (estimated from graph; not precise) NR NR No mention of tooth loss; sample size in subgroups not known; attachment level changes in shallow pocket look suspicious error? NR NR 20 NR NR NA 0.85 (estimated from graph; not precise) NR NR No mention of tooth lossample size in subgroups not known; NR NR 20 NR NR NA 0.7 (estimated from graph; not precise) NR NR No mention of tooth lossample size in subgroups not known; 5.84 0.32 23 NR NR SE 0.92 0.21 nr Smoking data presented for all tx groups combined (not usable) 7.3 1.9 One systemic AMX/Met and one placebo unavailable for 12month evaluation not reported not reported Not stated 0.4 (estimated from graph) not reported in graph N/A Confusing as to which data are site level and which are person level as well as which groups combined; The best data are in Figure 2; however, the data need to be read off the graph; data from Table 2 at the subject-level may be useful for the systemic (combining Emdo and no Emdo together) Not reported Not reported 1.21 0.43 nr not reported 8.22 (all pockets) 1.02 12 2.4 0.57 CAL(full mouth) Goodson, 2012 Mombelli 2005 site specific available in fig 3. (interproximal sites with baseline PPD >= 5 mm at 24 months: SRP+SMA=1.44 +/0.09; SRP=0.94+/0.09; SEM, n=95 and 92 respectively, must be no. sites) 29 (baseline mean only reported for those completing the trial) 12 month 3,6,12,18,24 4 sites per tooth on all teeth 12 months 10 days, as well as 2, 6, and 12 months after treatment. One site per quadrant with test or placebo gel in 2 groups using test or placebo systemic AMX/MET / ? One site measured per tooth (buccal) (Baseline data of those who completed also given) 6 months 3 months 146.4 13 subjects Mean change from baseline, CAL, mm Appears data given BY SITE 16 sites in 8 subjects RAL (for "qualifying" (PD ≥ 5mm and BOP sites) Ribeiro, 2009 5-6 mm pockets RAL (for "qualifying" (PD ≥ 5mm and BOP sites) July 2015 28 16 sites in 8 subjects not reported in graph (Baseline data of those who completed also given) 0.74 12 subjects Not reported Not reported SD 2.22 1.18 SD Not reported Not reported not reported Page 135 Systemic antimicrobials: amoxicillin-metronidazole + SRP - Updated literature Citation: Author, Year Outcome measure Miranda, 2014 CAL July 2015 Time period for No. Sites treated data presented in per mouth / No. this abstraction (as sites averaged per close to 9 months tooth as possible) 12 months not stated Test sample size Baseline Test mean Baseline 29 Full mouth: 4.6 Test Test SD or Mean Mean SD Test SD or SE sample Test mean at SE (list difference (or SE) (list value) size at end end of test value) at SD or SE? TEST (final- difference, Baseline of test period end of test baseline) TEST period period Full mouth: 1.2 27 Full mouth: 3.7 Full mouth: 0.9 disease sites: disease sites: SD for full PD 4-6mm: PD 4-6mm: mouth; SE for 1.36 0.10 (SE) diseased PD 7+mm: PD 7+ mm: sites 2.89 0.27 Control sample size Baseline Control mean Baseline Control SD or SE (list value) Baseline 29 Full mouth: 4.6 Full mouth: 0.8 Control Control SD Control sample or SE (list mean at end size at end value) at SD or SE? of test on test end of test period period period 23 Full mouth: 4.1 Full mouth: 0.9 Mean difference CONTROL (finalbaseline) Mean SD (or SE) Caries data difference, CONTROL disease sites: disease sites: SD for full PD 4-6mm: PD 4-6mm: mouth; SE for 0.77 0.10 Not reported diseased PD 7+mm: PD 7+mm: sites 1.78 0.26 Statstical analysis notes None Page 136 Systemic antimicrobials: metronidazole + SRP - Study characteristics Citation: Author, Year Country Palmer et al. Brit Dent J 1 998;184: 548-552. England Local metro (Elyzol) arm excluded Palmer et al. JCP 1999;26:158-163 England Smoker subset of 1998 paper. Haffajee 2007 U.S. (was in "other" group) Special population? (e.g. smokers) and other data regarding inclusion - exclusion criteria for patients and teeth 47 female and 43 males to yield 90 subject of which 28 were smokers. Inclusion criteria were: good general health, no antibiotics or periodontal therapy in previous 6 months, affected sites should have PD of ≥ 5 mm with bone loss and CAL (from CEJ) of 2 mm and 4 mm, respectively. 47 female and 43 males to yield 90 subject of which 28 were smokers. Inclusion criteria were: good general health, no antibiotics or periodontal therapy in previous 6 months, affected sites should have PD of ≥ 5 mm with bone loss and CAL (from CEJ) of 2 mm and 4 mm, respectively. α No at least eight sites with PD>4 mm / no perio tx within previous 3 months / not pregnant or nursing / no systemic conditions requiring antibiotics Severity of disease (e.g. refractory, mild, moderate) Test Control subject age subject age (mean, (mean, median, median, range as range as described) described) Control Dose/ duration/ frequency/ timing if applicable Control Test (typically adjunct) Test (typically adjunct) Dose/ duration/ frequency/ timing including simultaneous/ before/ after SRP + Syst Metro 200 mg, t.i.d., for 7 days Was standard counseling mentioned as part of control treatment? Trial design (split mouth / parallel group / cross over) Duration of study Adverse events reported OHI (disclosing tabs, brush, floss & interproximal brush) at baseline and 4 weeks later. Parallel group 6 months Not reported. OHI (disclosing tabs, brush, floss & interproximal brush) at baseline and 1 week following completion of SRP when all subjects received a polishing of teeth Parallel group 6 months Not Reported SRP Moderate to advanced chronic periodontitis stratified by severity and whether subject was a smoker or nonsmoker. 44.7±6.2 50.5 ± 6.1 Treated by RDH and consisted of OHI, ultrasonic subgingival scaling using anesthetic. Two appts of 90 minutes each1 week apart during which two contralateral quadrants were treated. Study did not use a placebo drug. The control group received SRP under local anesthetic only. SRP Moderate to advanced 44.7±6.2 Treated by RDH and consisted chronic periodontitis (Among 50.5±6.1 of OHI, ultrasonic subgingival stratified by severity smokers: (Smokers=50, scaling using anesthetic. Two and whether subject mean=42.1, nonappts of 90 minutes each1 was a smoker or non- among non- smokers=50.7) week apart during which two smoker. smokers=46) contralateral quadrants were treated. Chronic periodontitis 44 +/- 11 43 +/- 15 SRP at baseine and then at 3 maintenance SRP visits post baseline Smokers not excluded Study did not use a placebo drug. The control group received SRP under local anesthetic only. Article used both of the following terms: SRP and subgingival scaling. SRP was performed a quadrant at a time under local anaesthesia at approximately weekly intervals SRP + Syst Metronidazole SRP+Systemic Metronidazole 200 mg, t.i.d., for 7 days 250 mg t.i.d. for 14 days /started at first SRP visit No Parallel group 12 months One subject in the MET group reported dizziness and a second subject described diarrhoea associated with the use of the study medication. No adverse events were reported in the SRP-only group Systemic antimicrobials: metronidazole + SRP - Updated literature: Citation: Author, Year Preus, 2013 Country norway July 2015 Special population? (e.g. smokers) and other data regarding inclusion exclusion criteria for patients and teeth Severity of disease (e.g. refractory, mild, moderate) Excluded patients with prior Chronic perio (Also note that all systematic periodontal subjects went through prestudy treatment, systemic diseases hygiene phases-3 months- that known to be association with included OHI, supragingival scaling, perio, continuous medication fl varnish, desentsititizing TP and known to affect severity of only included if >=5 perio, allergies to sites with a PD >=5 mm should metronidazole remain. Test subject age (mean, median, range as described) Control subject age (mean, median, range as described) Control Control Dose/ duration/ frequency/ timing if applicable 3times day for 10 days starting the day before the second SRP session 56.8 +/-8.3 54.9 +/- 8.5 SRP, CHX rinse after SRP, Placebo All groups rinsed with CHX for 1 minute with 10 mL 0.2% CHX postSRP sessions (treatment and control) Test (typically adjunct) SRP, CHX rinse after SRP, and MET Test (typically adjunct) Was standard Trial design Dose/ duration/ frequency/ timing counseling (split mouth / Duration of including simultaneous/ before/ mentioned as part of parallel group study after control treatment? / cross over) 400 mg MET 3 times day for 10 days Yes, including CHX gel for 1 minute every night; rinse with a 0.2% CHX solution every morning for 9 days; parallel 12 months Adverse events reported Diarrhea, nausea,headache, feeling unwell, metallic taste, diziness / Although patients allocated to the two groups treated with metronidazole did report adverse effects more often than did patients treated with placebo, no statistically significant differences were observed between groups regarding the occurrence of adverse effects observed in conjunction with the intervention (Table 4), when comparing side effects in antibiotic groups (groups 1 and 3) against those in the placebo groups (groups 2 and 4). Page 137 Systemic antimicrobials: metronidazole + SRP - Outcomes data Citation: Author, Year Palmer et al. Brit Dent J 1 998;184: 548552. Outcome measure Other time No. Sites treated Time period for periods for per mouth / No. Test sample size data presented in which data sites averaged per Baseline this abstraction are available tooth Test mean Baseline Mean attachment level gain 6 months (24 weeks) Reported as mean number of teeth/pt and percentage of sites ≥ 4.6 mm at baseline. Control Group: 27.4 ± 2.4 Baseline, 8 and 24 weeks teeth and 19.33 ± 10.79 percent sites Syst Metro Group: 26.6 ± 3.6 teeth and 20.94 ± 10.08 percent sites Only reported attachment level gains - no baseline data 6 months Reported as mean number of teeth/pt. Used Florida probe at 6 sites/tooth. Smoker/Control 27.4 teeth/pt NonBaseline, 2 and 6 months smoker/Control 27.4 teeth/pt Smoker/Syst Metro 27.3 teeth/pt. Non-smoker/Syst Metro 26.3 teeth/pt Local metro (Elyzol) arm excluded Palmer et al. JCP 1999;26:158-163 mean change in CAL Smoker subset of 1998 paper. Not reported Test SD or SE (list value) Baseline Only reported attachment level gains - no baseline data Test sample size at end of test period Syst Metro Group: 31 pts (10 smokers) Smoker pts Not reported Not reported Test mean at end of test period Test SD or SE (list value) at end of test period Not reported Not reported Control SD or Mean gain TEST Mean SD (or SE) sample size SE? (Baseline-final) gain, TEST Baseline SD 10 Not reported Not reported Not reported SD Non-smoker 21 pts Probing AL, six sites per tooth at all teeth excluding third molars; measured to nearest mm using North Carolina probe At Baselenie 12 month data are the changes in AL from baseline Haffajee 2007 (was in "other" group) Syst Metro Group: 0.67 Reported as mean change in CAL. Smoker 0.43 Non-smoker 0.79 Control mean Baseline Only reported attachment Syst Metro Group: Not reported level gains ± 0.67 no baseline data Control SD or Control sample Control mean Control SD or SE SE (list value) size at end on test at end of test (list value) at end of Baseline period period test period Only reported attachment level gains - no baseline data Smoker ± 0.57 Non-smoker ± 0.70 Not reported Not reported Not reported 27 pts (9 smokers) Not reported . Smoker Control 9 pts Non-smoker Not reported Control 18 pts Not reported Not reported SD or SE? SD Mean gain CONTROL (baseline-final) Mean SD (or SE) Caries gain, CONTROL data 0.51 ± 0.43 12 months Mean changes in AL in sites with baseline PD>6mm 3 and 6 months (read off figure, final - baseline) -0.39 3.21 0.78 24 Not reported Not reported SE Sites>6mm: -2.4 [Mean percent of sites exhibiting gain/loss of AL>2mm also available at all time points] (Not stated, all data: estimated dropouts, but off figure 1 as 0.1 the means are given (sites>6mm) only for those estimated off who Figure 2 as 0.4 completed the study) = Use these for SD 23 calculations for all txs SD all data: estimated off figure 1 as 0.1 (read off figure, finalbaseline) 3.03 0.56 23 Not reported Not reported Only sites with baseling PD of ≥ 4.6 mm (as measured by Florida probe) were evaluated. For frequency distribution, sites were categorized as ≥ 4.6 mm and ≥ 7 mm. Data were analyzed using analysis of variance. Power of test was performed using the formula of Armitage and Berry. Probing depths and attachment levels were recorded to the nearest 0.2 mim at six points around all teeth with a Florida (Florida Probe Corporation, Gainesville, Florida, USA) computerised constant force probe using the pocket and disk probes Only sites with baseline PD ≥ 4.6 mm were evaluated (equivalent to 5 mm using a manual probe). Mean PD and Reported as mean Smoker Control change in CAL were calculated. Variables with skewed change in CAL. ± 0.46 distributions were log transformed prior to analysis. Post-Tx Smoker Control Not data were analyzed for differences using factorial analysis of 0.47 Reporte Non-smoker variance with equivalent baseline values as covariates. Non-smoker d Control Multiple linear regression analysis was applied to data with Control PD at 6 months as the dependent variable and smoking ± 0.43 0.53 status, antimicrobial therapy and severity of disease as independent variables. Bars are unclear; (Not stated, dropouts, but the full mouth not noted means are given how many only for those who completed the study) = 24 Not reported Statstical analysis notes SE (sites>6mm) estimated off Figure 2 as 0.4 -0.15 Sites>6mm: -1.2 Use these for SD calculations for all txs N/A Examiners formally calibrated / difference among groups at each time point was sought using ANCOVA adjusting for baseline values. / If significant differences were found among the four groups, significance of differences between pairs of treatment groups was determined and the Bonferroni adjustment was used to adjust for multiple comparisons. / power calculation with reasonable values for SD and clinically signficant results Systemic antimicrobials: metronidazole + SRP - Updated literature Citation: Author, Year Outcome measure Preus, 2013 CAL July 2015 Time period for No. Sites treated data presented in per mouth / No. this abstraction (as sites averaged per close to 9 months tooth as possible) 12 months 4 sites per tooth: mesial, buccal, distal, and lingual surfaces Test sample size Baseline Test mean Baseline 46 2.06 Test Test SD or Mean Mean SD Test SD or SE sample Test mean at SE (list difference (or SE) (list value) size at end end of test value) at SD or SE? TEST (final- difference, Baseline of test period end of test baseline) TEST period period 1.11 45 1.21 0.73 sd 0.81 (0.67 to 0.96) Control sample size Baseline Control mean Baseline Control SD or SE (list value) Baseline 47 1.91 1.07 Control Control SD Control sample or SE (list mean at end size at end value) at SD or SE? of test on test end of test period period period 46 1.29 0.8 sd Mean difference CONTROL (finalbaseline) Mean SD (or SE) Caries data difference, CONTROL 0.64 (0.52 to 0.76) not reported Statstical analysis notes None Page 138 Systemic antimicrobials: azithromycin + SRP - Study characteristics Citation: Author, Year Country Special population? (e.g. smokers) and other data regarding inclusion - exclusion criteria for patients and teeth Severity of disease (e.g. refractory, mild, moderate) Test subject age (mean, median, range as described) Control subject age (mean, median, range as described) Control Control Dose/ duration/ frequency/ timing if applicable 51.0+/–8.8 years SRP (quadrant) Conventional SRP, 4-6 sessions with an approximately 1 week interval; All subjects received a professional mechanical tooth cleaning once a week by dental hygienists after treatment. SRP (full mouth) plus systemic azithromycin 43 +/- 15 SRP at baseine and then at 3 maintenance SRP visits post baseline SRP was performed a quadrant at a time under local anaesthesia at approximately weekly intervals SRP+Systmic Azithromycin SRP (no placebo) 2 sessions of SRP using ultrasonic scalers and hand instruments SRP+ CHX rinse + placebo using an ultrasonic device and hand curettes / two 1.5-h appointments, within 7 days / and the adjunctive use of a 0.12% chlorhexidine and 0.05% cetylpyridiniumchloride rinse (Perio AidTratamientos, Dentaid) was prescribedtwice a day for 15 days, with 15 ml for30 s, immediately after rinsing withwater after brushing /one placebo per day Test (typically adjunct) No (smokers excluded) Gomi 2007 Haffajee 2007 Japan U.S. an average PD of >4 mm with BOP; and radiographic evidence of alveolar bone loss in each quadrant / no systemic or topical antibiotic treatment in the preceding 3 months No at least eight sites with PD>4 mm / no perio tx within previous 3 months / not pregnant or nursing / no systemic conditions requiring antibiotics Severe chronic periodontitis Chronic periodontitis 45.4 +/ – 14.3 years 47 +/- 14 Smokers not excluded ONLY SMOKERS, ≥1 pack per day of cigarettes for more than 5 years Mascarenhas 2005 U.S. at least five sites with probing depths (PD) of ≥5 mm with bleeding on probing (BOP) (Methods say at least 6 sites) moderate to severe chronic periodontitis 47 ± 10.06 (33- 45.34 ± 10.75 64* Range (31-66* Range No (list stratified for smokers (>10 cigarettes per day) and non-smokers (non-smokers, former smokers or smokers of <10 per day) / Smokers included and analyzed as covariate but "could not be properly assessed" Oteo 2010 Spain untreated moderate chronic periodontitis (Armitage 1999) with radiographic moderate chronic evidence of generalized alveolar bone loss >30%, periodontitits (severe (iii) presence of at least one pocket with PPD perio EXCLUDED) >5mm per quadrant with bleeding on probing (BoP) / no perio tx in last 3 years / NO severe periodontitis with more than one tooth with a site with PPD>7mm per quadrant except if it was scheduled for extraction / no antibiotics in previous month / not lactating or pregnant / no chronic diseases / no use of NSAIDs / must have P. gingivalis 46.6, range 38–62 47.1, range 36–65 No (5 smokers in each group initially) Sampaio 2011 Brazil at least 15 teeth (excluding third molars) and at least 30% of the sites with PD and CAL≥5mm and bleeding on probing (BOP), a minimum of three non-contiguous inter-proximal sites with PD and CAL≥7mm and two other non-contiguous inter-proximal sites with PD and CAL≥6 mm / previous subgingival periodontal therapy, pregnancy, nursing, systemic diseases that could affect the progression of periodontal disease, longterm administration of anti-inflammatories, need for antibiotic coverage for routine dental therapy, antibiotic therapy in the previous 6 months and allergy to AZM. Generalized chronic periodontitis 44.40 ± 7.42 43.52 ± 5.90 Test (typically adjunct) Dose/ duration/ frequency/ timing including simultaneous/ before/ after Was standard counseling mentioned as part of control treatment? Trial design (split Duration mouth / of study parallel group) Adverse events reported After screening, all subjects received 3 days prior to full mouth SRP, 10/17 people in test group took analgesic after two or three visits of azithromycin taken once per day for 3 treatment (control group not reported); 1/17 in test toothbrushing instructions days / full mouth SRP performed group complained of mild diarrhea / In addition, (TBIs) including the use using curets for about 90 minutes / All three subjects (17.6%) complained of of interproximal cleaning Parallel group 25 weeks subjects received a professional hypersensitivity caused by gingival recession aids such as floss and mechanical tooth cleaning once a within 1 month after treatment; however, it was a interdental brushes, week by dental hygienists after mild degree, and there was symptomatic relief depending on treatment. within 1 month. individual needs, and supragingival scaling 500 mg once daily for 3 days / started at first SRP visit No Parallel group 12 months Two subjects in the AZ group reported adverse events: one had an allergic reaction to the study medication and the second had difficulty swallowing the tablets, although this latter subject completed the course of medication No adverse events were reported in the SRP-only group Placebo once a day for 5 days / fullmouth SRP performed under local anaesthesia in four to six appointments of approximately 2 h each. The treatment of the entire oral cavity was completed in a maximum period of 2 weeks using mainly manual SRP + placebo instruments / The end point for each SRP appointment was ‘‘smoothness of the scaled roots’’, which was checked by the study coordinator / The placebo therapy started immediately after the last session of mechanical instrumentation SRP + systemic azithromycin SRP+CHX rinse+ systemic azithromycin Systemic azithromycin (+SRP) the AZT + SRP (test) group received the study drug for 5 days (two 250 mg tablets the first day and one 250 mg tablet for each of the next 4 days). Tablets were taken 1 hour before or two hours after a meal. SRP+CHX rinse as per control / one 500 mg per day for 3 days Pts advised not to use mouthwash or rinse during study period / OHI at both SRP appts followed by demonstrations parallel group 6 months Not reported At baseline visit, standardized oral hygiene instructions were explained, including the use of a manual toothbrush and interParallel group dental brushes (Vitis Access softs and Interproxs, Dentaid). / Oral hygiene instructions were reinforced at each visit of SRP 6 months One patient in the test group reported diarrhoea probably associated with the study medication. No adverse events were reported in the control group. 1 year On the last day of medication subjects answered a questionnaire about any self-perceived sideeffects of the medication/ placebo. / Adverse events were reported by four subjects from the test group and three from the control group, including diarrhoea (test group, n=2), headache or dizziness (test group, n=1; control group, n=2), excessive sleepiness (n=3 per group); metallic taste (test group, n=2; control group, n=3) and general unwellness (n=1 per group). All subjects reported that the medications did not cause any major disturbance in their daily routine and that they would start the treatment again if necessary. During the initial phase, all subjects received 500 mg/day for 5 days / The antibiotic instruction on proper therapy started immediately homecare techniques after the last session of mechanical and were given the same instrumentation dentifrice (Colgate Total) to use during the study period Parallel AZM 3 days, then FM SRP in one visit or PM SRP in 3 visits. No smokers Yashima 2009 Japan July 2015 ≥20 remaining teeth; average probing depth (PD) ≥4 mm, with bleeding on probing (BOP) and deepest PD ≥6 mm; unremarkable general health based on medical history and clinical judgment; and radiographic evidence of alveolar bone loss in each quadrant / no use of systemic and topical antibiotic treatment within 3 months prior to the start of the study; no subgingival instrumentation within 12 months prior to the start of the study; no systemic illness or medicationvwith periodontal manifestations; no pregnancy; and no smoking. FMSRP+AZM: 51.1 ± 11.6 years Chronic periodontitis PMSRP+AZM: 50.8 ± 14.2 years 51.0 ± 10.6 years CSRP conventional SRP FMSRP+AZM: azithromycin, 500 mg once a day for 3 days, before After screening, full-mouth SRP was performed / Full- all subjects received two mouth SRP was performed using a or three sessions of surgical loupe, Gracey curets, toothbrushing and ultrasonic instruments under local instructions (TBI), anesthesia in a single visit. The including the use of Conventional SRP was performed in average treatment time was ~120 interproximal six sessions, with an interval of 1 week Systemic minutes. cleaning aids, depending between sessions, azithromycin on the individual Parallel group 12 months using a surgical loupe, Gracey curets, (+SRP, either full needs, and supragingival and ultrasonic or partial mouth) PMSRP+AZM: After taking the scaling. / After treatment, instruments under local anesthesia. medication, SRP of the whole mouth all subjects received was completed in three sessions within professional 7 days, during the effective half-life of mechanical tooth systemically administered cleaning once a month, azithromycin. SRP was performed which was performed by using a surgical loupe, Gracey curets, dental hygienists and ultrasonic instruments under local anesthesia. It took ~30 to 40 minutes for each session (one-third of the full mouth/session). 1 FM-SRP (+azithromycin) complained of diarrhea Page 139 Systemic antimicrobials: azithromycin + SRP - Updated literature Citation: Author, Year Country Special population? (e.g. smokers) and other data regarding inclusion exclusion criteria for patients and teeth Severity of disease (e.g. refractory, mild, moderate) Test subject age (mean, median, range as described) Control subject age (mean, median, range as described) Control Control Dose/ duration/ frequency/ timing if applicable Test (typically adjunct) Han, 2012 Turkey Excluded: severe medical disorders including diabetes, immunologic disordyers, any history of systemic disease, pregnancy, smoking >10 cigarettes per day severe generalized chronic periodontal disease 46.8 +/- 5.1 44.8 +/- 5.0 Placebo capule 1x day for 3 days azithromycin 500mg 1x day for 3 days Yes parallel 6 months none reported Martande, 2014 India Exclusion: systemic diseases such as diabetes, smokers, systmic antibiotics w/I prior 3 months, pregancy, SRP or perio tx in prior 6 months, immunocompromised Moderate to severe periodontitis harboring S. Actinomycetemcomitans 32.6 (+/- 5.4) 33.3 (+/- 7.3) Placebo capule Placebo pill 1xday for 3 days 500mg AZ 1x day for 3 days Yes, OHI parallel 12 months not assessed July 2015 Test (typically adjunct) Was standard Trial design Dose/ duration/ frequency/ timing counseling (split mouth / Duration of including simultaneous/ before/ mentioned as part of parallel group study after control treatment? / cross over) Adverse events reported Page 140 Systemic antimicrobials: azithromycin + SRP - Outcomes data Citation: Author, Year Outcome measure Gomi 2007 CAL Haffajee 2007 Probing AL, six sites per tooth at all teeth excluding third molars; measured to nearest mm using North Carolina probe At Baselenie Time period for data Other time Test sample presented in this periods for No. Sites treated per mouth / size abstraction (as close to which data are No. sites averaged per tooth Baseline 9 months as possible) available 25 weeks Test SD or SE (list value) Baseline Test sample size at end of test period Test mean at end of test period Test SD or SE (list value) at end of test period SD or SE? Mean gain TEST (Baselinefinal) 7.47 1.96 17 4.85 1.05 SD Not reported Mean SD (or Control sample Control mean SE) gain, size Baseline TEST Baseline Not reported Not reported N/A Subject unit of analysis; PD and CAL primary outcomes measures; unpaired t-tests for improvement within groups baseline to posttreatment; evel of significance set at 0.05 N/A Examiners formally calibrated / difference among groups at each time point was sought using ANCOVA adjusting for baseline values. / If significant differences were found among the four groups, significance of differences between pairs of treatment groups was determined and the Bonferroni adjustment was used to adjust for multiple comparisons. / power calculation with reasonable values for SD and clinically signficant results 5.02 1.5 15 (read off graph) 4.0 Judged (read off graph) to be SD 1.2 although stated SE 1.13 Not given 16 3.92 0.97 15 (read off graph) 3.5 (read off graph) 1.0 Judged to be SD although stated SE 0.46 Not given N/A 15 (read off graph) 3.6 (read off graph) 1.1 15 (read off graph) 3.1 Judged (read off graph) to be SD 1.1 although stated SE 0.55 Not given 16 (read off graph) 2.8 (read off graph) 0.6 15 (read off graph) 2.7 (read off graph) 0.8 Judged to be SD although stated SE 0.11 Not given N/A CAL, Moderate sites (baseline PD 4 to 6 mm). 15 (read off graph) 5.5 (read off graph) 1.3 15 (read off graph) 4.0 Judged (read off graph) to be SD 1.4 although stated SE 1.52 Not given 16 (read off graph) 4.9 (read off graph) 0.5 15 (read off graph) 4.0 (read off graph) 0.9 Judged to be SD although stated SE 1.01 Not given N/A CAL, Deep sites (baseline PD >6 mm). 15 (read off graph) 7.8 (read off graph) 1.1 15 (read off graph) 5.1 Judged (read off graph) to be SD 1.1 although stated SE 2.56 Not given 16 (read off graph) 7.8 (read off graph) 0.6 15 (read off graph) 6.4 (read off graph) 1.5 Judged to be SD although stated SE 1.32 Not given N/A CAL, Shallow sites (baseline <4 mm). Mascarenhas 2005 6 months Oteo 2010 CAL 6 months 3 months 1, 3 months clinical attachment level (CAL), and bleeding on probing (BOP) was assessed for all teeth / CAL measured at six sites per tooth PPD and recession (REC) in millimetres at six sites per tooth in all teeth, excluding third molars. CAL calculated as the sum of PPD and REC 15 patients SE (Not stated, dropouts, but the (sites>6mm) means are given estimated off only for those who Figure 2 as 0.4 completed the study) = 23 Use these for SD calculations for all txs -0.19 Sites>6mm: -1.7 3.46mm 0.18 15 2.7 0.18 SE 0.96 SD 15 CAL (all sites) Not reported 5.74 Statstical analysis notes 3.42 Not reported 17 Caries data full mouth not noted how many 25 1.37 Mean SD (or SE) gain, CONTROL all data: estimated off figure 1 as 0.1 (read off figure, finalbaseline) 0.88 7.21 Mean gain CONTROL (baselinefinal) (Not stated, dropouts, but the means are given only for those who completed the study) = 25 3 and 6 months 17 SD or SE? 17 12 month data are the changes in AL from baseline Not reported Control SD Control Control SD or Control mean or SE (list sample size SE (list value) at end of test value) at end on test at end of test period Baseline period period Not described 12 months 5 and 13 weeks Test mean Baseline 0.76mm 0.12mm 14 all data: estimated off figure 1 as 0.1 (read off figure, finalbaseline) 3.03 0.56 23 Not reported Not reported SE (sites>6mm) estimated off Figure 2 as 0.4 -0.15 Sites>6mm: -1.2 3.56 0.31 13 3.32 0.33 SE Use these for SD calculations for all txs 0.28mm 0.14mm N/A Data were first averaged within each patient, and then patient means were analyzed between treatment groups to determine baseline comparability of the two groups. Differences between study drug and control groups were performed using the Student t test Intra-examiner calibration was performed twice, before and during the study / evaluating the normality of the distribution / ANCOVA was selected to compare both groups, either at baseline or in changes baseline follow-up visits (inter-group comparison), including baseline values of the examined variable, smoking and gender as cofactors / Bonferroni correction was used / PPD primary outcome and used for power calculations Baseline versus 6 months Placebo 0.28 0.14 0.00 0.57 0.016 Baseline CAL, smoking Test 0.76 0.12 0.52 1.01 Full mouth: 1.02 Sampaio 2011 CAL (distance in mm from the cementoenamel junction to the bottom of the sulcus/pocket) 12 months 6 months measured at six sites per tooth in all teeth, excluding the third molars./ The PD and CAL measurements were recorded to the nearest millimetre using a North Carolina periodontal probe Full mouth: 1.62 Full mouth: 1.04 Initially shallow Initially shallow sites (PD≤3 sites (PD≤3 mm): mm): 0.17 0.94 20 5.51 0.94 19 but 20 analyzed using LOCF 4.44 0.77 SD Initially deep - Full mouth: 1.65 Initially shallow Initially shallow sites (PD≤3 sites (PD≤3 mm): mm): 1.10 0.17 20 Initially Initially intermediate intermediate sites (PD 4- 6 sites (PD 4- 6 mm): mm): - 1.14 1.16 Initially deep July 2015 - 5.74 0.83 19 but 20 analyzed using LOCF 4.69 0.89 SD Initially Initially intermediate sites intermediate (PD 4- 6 mm): sites (PD 4- 6 1.31 mm): -1.15 Initially deep sites (PD≥7mm): Initially deep 1.70 N/A power based on identying 1 mm difference in CAL in sites with initial PD >= 7 mm and assuming SD of 1 mm / The inter-examiner variability was 0.25mm for PD and 0.29mm for CAL. The mean intraexaminer variability was 0.22mm (PD) and 0.27mm (CAL) for the first examiner (M. R.) and 0.23mm (PD) and 0.26mm (CAL) for the second examiner (E. S.). One examiner carried out all clinical measurements in a given subject and treatment was performed by the second clinician / Intention-to-treat analyses were performed in these two subjects (their 6-month data were carried forward). / also looked at change in CAL over time intervals by pocket depth Page 141 Systemic antimicrobials: azithromycin + SRP -Updated literature Time period for No. Sites treated data presented in per mouth / No. this abstraction (as sites averaged per close to 9 months tooth as possible) Citation: Author, Year Outcome measure Han, 2012 CAL 6 months Martande, 2014 CAL 12 months July 2015 Test Test SD or Mean Mean SD Test SD or SE sample Test mean at SE (list difference (or SE) (list value) size at end end of test value) at SD or SE? TEST (final- difference, Baseline of test period end of test baseline) TEST period period Test sample size Baseline Test mean Baseline not stated 18 5.68 0.7 14 not reported not reported SD 1.55 not stated 35 7.69 1.02 35 4.97 1.18 SD 2.71 Control Control SD Control sample or SE (list mean at end size at end value) at SD or SE? of test on test end of test period period period Mean difference CONTROL (finalbaseline) Control sample size Baseline Control mean Baseline Control SD or SE (list value) Baseline 0.5 18 5.32 1 14 not reported not reported SD 1.54 0.4 not reported None 1.15 35 7.63 1.42 35 5.91 1.09 SD 1.71 1.29 not reported None Mean SD (or SE) Caries data difference, CONTROL Statstical analysis notes Page 142 Systemic antimicrobials: clarithromycin + SRP - Study characteristics Special population? Citation: (e.g. smokers) and other data regarding Country Author, Year inclusion - exclusion criteria for patients and teeth Severity of disease (e.g. refractory, mild, moderate) Control Test subject subject age age (mean, (mean, median, median, range as range as described) described) Control Control Dose/ duration/ frequency/ timing if applicable Test (typically adjunct) Was standard Trial design Duratio Test (typically Dose/ duration/ frequency/ counseling (split mouth / n of adjunct) timing including simultaneous/ mentioned as part of parallel group study before/ after control treatment? / cross over) Adverse events reported No Pradeep 2011 India Systemically healthy patients having at least 20 natural teeth with a PD of ≥ 5 mm and CAL of ≥ 3 mm in at least 30% sites were recruited / Patients with any antimicrobial therapy or non steroidal anti-inflammatory drugs or steroidal therapy or patients with any periodontal therapy in the preceding 6 months, having known or suspected allergy to macrolide group, smokers (current or past), or subjects having tobacco in any other form, alcoholics, and pregnant or lactating females were excluded from the study Chronic periodontitis 35.2 ± 6 years 37.3 ± 5.7 years SRP + placebo placebo 500 mg b.i.d. for 3 days / SRP (Ultrasonic) was performed at baseline and at 6 months (as SRP+ prophylactic systemic measure during Clarithromycin maintenance phase), until the root surface was considered thoroughly smooth and clean by the operator. 500 mg b.i.d. for 3 days No anti-plaque agent was prescribed after treatment, however home care oral hygiene like brushing twice a day and rinsing after every meal was recommended Parllel group 9 months No adverse reaction was observed in any subject from the test group, and no patient reported any discomfort (except for unpalatable taste and mild gastric intolerance as reported by 2 patients). Systemic antimicrobials: clarithromycin + SRP - Outcomes data Citation: Author, Year Outcome measure Reduction in CAL compared to Baseline, mm Pradeep 2011 UNC-15 graduated periodontal probe was used to standardize the measurement of PD and CAL July 2015 Other time Time period for periods for No. Sites treated per Test sample data presented which data mouth / No. sites averaged size in this are per tooth Baseline abstraction available 6 months NOTE: SRP repeated at 6 months so 9month data ineligible CAL were assessed in all patients for all the teeth (except 3rd molars and teeth with orthodontic appliance) and in all quadrants at six locations 1, 3, 6, and around the tooth (mesio9 months buccal, buccal, disto-buccal, mesiolingual, lingual, and disto-lingual) as per UCLA charting. The PD and CAL values were estimated to their nearest millimetre Test mean Baseline Test SD or SE Test sample (list value) size at end of Baseline test period Test SD or SE Test mean at (list value) at SD or end of test end of test SE? period period Mean gain Control SD Control Control SD or Mean gain Mean SD (or Control sample Control mean TEST Control mean or SE (list sample size SE (list value) CONTROL SE) gain, size at end of test SD or SE? (BaselineBaseline value) at end on test at end of test (baselineTEST Baseline period final) Baseline period period final) Mean SD (or SE) gain, CONTROL Caries data Statstical analysis notes Subject based analysis 20 Not reported Not reported 18 Not reported Not reported SD 1.81 0.36 20 Not reported Not reported 19 Not reported Not reported SD 0.86 0.23 N/A 90% power; 2 sided at 90% power, with alpha error of 5% and standard deviation (SD) of S1 = 0.010, S2 0.1093 with a mean difference of 0.091 and an effect size of 1.527 r = 0.92 for duplicate CAL measurements Page 143 Systemic antimicrobials: moxifloxacin + SRP - Study characteristics Citation: Author, Year Guentsch 2008 Country Control Special population? Test subject Severity of disease subject age (e.g. smokers) and other data age (mean, (e.g. refractory, mild, (mean, regarding inclusion - exclusion median, range moderate) median, range criteria for patients and teeth as described) as described) Germany No attachment loss >=5 mm at >30% of sites and age >=35 years. / no systemic condition / at least 5 pockets >= 5mm in different quadrants / radiographic evidence of moderate to advanced periodontal destruction / no antibiotics, steroids, NSAIDs within previous 6 months / no peridontal treatment in previous 12 months / not pregnant or nursing / Less than 35% of the subjects were active smokers Severe chronic periodontitis Control Dose/ duration/ frequency/ timing if applicable Control Test (typically adjunct) Dose/ duration/ Test (typically frequency/ timing adjunct) including simultaneous/ before/ after Was standard counseling mentioned as part of control treatment? Trial design (split mouth / Duration of parallel group study / cross over) Treatment started with chlorhexidine rinsing Before the onset (0.12%) for 1minute. Subgingival depuration of the study, the plaque index had and root planing for disrupting the subgingival to be <35% after the hygiene biofilm were performed in all four quadrants phase, which included under local anesthesia as a fullmouth systemically supragingival calculus removal procedure within 24 hours by the same SRP+Systemic mean age of 49.6 – administered MOX, 400 and oral hygiene instructions / SRP No Placebo experienced periodontist (one calibrated moxifloxacin at Parallel group 9.4 years at 12 months (92 pts) mg once daily for 7 days each center performed the tx in the periodontist at each center). SRP was antibiotic dose (MOX group). same standardized manner; performed with manual instruments# on all subjects received OHI from teeth. An ultrasonic instrument** was only experienced periodontist / all pts used in the furcation areas. SRP was received SPT and reinforcement of completed within 120 minutes. The endpoint OHI at 3-month intervals of SRP was a tactile smooth root surface. Adverse events reported None of subjects in antibiotic groups reported any adverse event at any time point associated with the therapy 12 months They dropped out subjects who needed to be retreated. - a lot more in the control group Systemic antimicrobials: moxifloxacin + SRP - Outcomes data Citation: Author, Year Outcome measure Time period for data presented in this abstraction Guentsch 2008 CAL To nearest mm at 6 sites per tooth 12 months July 2015 Other time No. Sites treated per periods for which mouth / No. sites averaged data are available per tooth 3 and 6 months full mouth not noted how many Test sample size Baseline Test mean Baseline 37 (people) 5.5 Test SD or Test SD or Test sample Test mean at SE (list SE (list size at end of end of test value) at value) test period period end of test Baseline period 0.94 35 3.58 0.51 SD or SE? Mean gain TEST (Baselinefinal) Mean SD (or SE) gain, TEST Control sample size Baseline Control mean Baseline SD 1.79 0.52 28 5.15 Control SD or Control SD or Control sample Control mean at SE (list value) SE (list value) size at end on test end of test SD or SE? at end of test Baseline period period period 0.55 21 3.61 0.57 SD Mean gain CONTROL (baseline-final) Mean SD (or SE) gain, CONTROL Caries data Statstical analysis notes 1.48 0.53 N/A Calibration, reliability, power calculations; normal distributions verified with clinical data Page 144 Systemic antimicrobials: tetracyclines + SRP - Study characteristics Citation: Author, Year Al-Joburi, 1989 Guentsch 2008 Lindhe 1983 Ng, 1998 Ramberg, 2001 July 2015 Country Special population? (e.g. smokers) and other data regarding inclusion - exclusion criteria for patients and teeth Canada 1. At least 35 years of age. 2. Both clinical and radiographie evidence of periodontal disease. 3. At least two sites with probing depth of at least 7 mm. 4. Presence of at least 15 teeth. 5. Absence of other serious or complicated oral pathosis. 6. No periodontal therapy within the past 6 months. 7. No antibiotic therapy within the past 6 months. 8. No contra-indications to participation (including hypersensitivity to the medications). 9. Not pregnant or nursing. Severity of disease (e.g. refractory, mild, moderate) Advanced adult chronic perio Presence of at least 2 sites with probing depth of at least 7 mm Severe chronic periodontitis Sweden The patients were in good general health, none of the women was aware of being pregnant, no patient had received antibiotics in the previous 6 months and none had a history of allergy to tetracycline. Each of the participants had at least 20 remaining teeth and 4 pairs of diseased sites (selected sites) around contralatera] premolars and incisors where pockets could be probed to >6 mm and where >4fl% of the alveolar bone had been lost. advanced periodontal disease. At least 20 remaining teeth and 4 pairs of diseased sites around contralateral premolars and incisors where pockets could be probed to ≥6mm and where ≥40% of the alveolar bone had been lost USA 15 current smokers, 17 non-smokers, not asked about past smoking / 1) radiographic bone loss greater than 50% in at least 2 matching tooth types per subject and 2) periodontal probing depth ≥5 mm in 1 or more sites in at least 2 matching tooth types. Exclusion criteria included: 1) systemic diseases that may affect periodontal status of the patient; 2) serious medical complications that may affect the treatment provided; 3) known side effects to either doxycy-cline or Ibuprofen; 4) periodontal therapy within 6 months from the onset of the study; and 5) antibiotic or nonsteroidal anti-inflammatory drug therapy within the last 6 months. Smokers were included but their data was not tracked separately. generalized periodontitis Sweden Sample not split: average age of those completing trial was 46 +/- 0.9 years Control Control Dose/ duration/ frequency/ timing if applicable Test (typically adjunct) SRP with placebo SRP started at baseline appt and completed in 2 sessions of 3 hours each performed within 1 week of each other / Placebo capsules every 6 hours for 14 days SRP + tetracycline Test (typically adjunct) Dose/ duration/ frequency/ timing including simultaneous/ before/ after tetracycline; 250 mg capsules every 6 hours for 14 days tetracycline; 250 mg capsules every 6 hours for 14 days Was standard counseling mentioned as part of control treatment? No Trial design (split mouth / parallel group / cross over) Parallel group Duration of study 24 weeks Adverse events reported Adverse reactions to drugs were assessed at 1- and 2-week visits by means of a questionnaire and blood analyses 1 tetracycline group severe diarrhea tetracycline; 250 mg capsules every 6 hours for 14 days No attachment loss >=5 mm at >30% of sites and age >=35 years. / no systemic condition / at least 5 pockets >= 5mm in different quadrants / radiographic evidence of moderate to advanced periodontal destruction / no antibiotics, steroids, NSAIDs within previous 6 months / no peridontal treatment in previous 12 months / not pregnant or nursing / Less than 35% of the subjects were active smokers Germany Test subject Control subject age (mean, age (mean, median, median, range range as as described) described) mean age of 49.6 – 9.4 years at 12 months (92 pts) 37-52 years of age 37-52 years of age range:32-72 60% were smokers in test, 56% in control.All women. tetracycline on the outcome of periodontal therapy, 35 adult subjects, 19 female and 16 male, aged between 24 and 60 years were included in a test group. They were recruited from a larger group of patients who in 1982 – 1984 were referred to the Department of Periodontology, Helsingborg, Sweden for treatment of advanced periodontal disease. Advanced Chronic 80 age and gender matched subjects from the same pool were Periodontitis. PPD Mean: 41.2 during the corresponding time interval recruited for a control group stratified: those <3 mm, (range:24-60) (42 female and 38 male). In order to be included in the study a 4-6mm, and >7mm subject had to be ±20 years of ge and have at least 16 teeth out of which at least 2 must be molars. Subjects were excluded (i) with systemic conditions which required antibiotic coverage for nonsurgical periodontal procedures, (ii) were pregnant, (iii) allergic to tetracycline or (iv) had taken antibiotic during the previous 6 months. Mean:42.1 (range:23-66) Treatment started with chlorhexidine rinsing (0.12%) for 1minute. Subgingival depuration and root planing for disrupting the subgingival biofilm were performed in all four quadrants under local anesthesia as a fullmouth procedure within 24 hours by the same experienced periodontist (one SRP No Placebo calibrated periodontist at each center). SRP was performed with manual instruments# on all teeth. An ultrasonic instrument** was only used in the furcation areas. SRP was completed within 120 minutes. The endpoint of SRP was a tactile smooth root surface. SRP+Systemic doxycycline at antibiotic dose Before the onset of the study, the plaque index had to be <35% after the hygiene phase, which included supragingival calculus orally administered removal and oral hygiene instructions / DOX,i 200 mg on the first each center performed the tx in the day followed by 100 mg/ day same standardized manner; subjects for 9 days (DOX group); received OHI from experienced periodontist / all pts received SPT and reinforcement of OHI at 3-month intervals Parallel group 12 months None of subjects in antibiotic groups reported any adverse event at any time point associated with the therapy They dropped out subjects who needed to be retreated. - a lot more in the control group SRP by quadrant SRP in 2 quadrants. Tetracycline hydrochloride Tetracycline daily for first 2 weeks 250 mg 4x per day. In the final 48 weeks, 250 mg once a day yes parallel 50 weeks no SRP+ placebo Scaling and root planing was performed under local anesthesia using ultrasonic and hand instruments; placebo one capsule per day SRP+DOXY 200mg doxycycline hyclate tablet orally the first day, 100mg per day for 6 weeks no Parallel group for this part of study 6 months This study did not collect data on adverse events SRP 4-6 sessions of scaling and root planing without antibiotic. 2% Chlorhexidene rinses b.i.d. during basic therapy (assumed to be 3 weeks) SRP plus systemic tetracycline 250mg tetracycline hydrochlorine (Tetracyklin NM Pharma) 4x a day for 3 weeks. SRP was done during this 3 week period. Chlorhexidene rinses b.i.d. during basic therapy (assumed to be 3 weeks) all subjects received oral hygiene instruction (OHI) Parallel group 13yrs Not reported Page 145 Systemic antimicrobials: tetracyclines + SRP - Updated literature Citation: Author, Year Country Special population? (e.g. smokers) and other data regarding inclusion exclusion criteria for patients and teeth Severity of disease (e.g. refractory, mild, moderate) Test subject age (mean, median, range as described) Control subject age (mean, median, range as described) Control Control Dose/ duration/ frequency/ timing if applicable Test (typically adjunct) Tsalikis, 2014 Greece Type 2 diabetes. Excluded: Type 1 diabetes, smoker, pregnant/lactating Moderate to severe periodontal distase 62.9 +/- 10 57.94 +/- 8.22 Placebo and SRP "Same instructions" as test. Assume this is 2 pills on day 1, and then 1 pill daily for 20 days SRP and DOX July 2015 Test (typically adjunct) Was standard Trial design Dose/ duration/ frequency/ timing counseling (split mouth / Duration of including simultaneous/ before/ mentioned as part of parallel group study after control treatment? / cross over) 200 mg loading dose and 100 mg for 20 days yes parallel 6 months Adverse events reported not reported Page 146 Systemic antimicrobials: tetracyclines + SRP - Outcomes data Citation: Author, Year Outcome measure Time period for data presented in this abstraction Other time periods for which data are available No. Sites treated per mouth / No. sites averaged per tooth Test sample size Test mean Baseline Baseline Pockets 1-3 mm deep Pockets 4-6 mm deep Al-Joburi, 1989 24 weeks 2, 18, 12 weeks 2 sites per patient / Williams 96 total patients to probe to nearest mm / according start; 32 per group to Clark et al procedure Pockets greater than 7 mm deep Test SD or SE (list value) Baseline CAL:7.25m m 0.46 CAL:9.06m m 0.26 Test sample size at end of test period Test mean at end of test period Test SD or SE (list value) at end of test period 7.06mm 0.55 8.00mm 0.27 28 patients CAL:10.58 0.25 SD or SE? Mean gain TEST (Baseline-final) Mean SD (or SE) gain, TEST 0.26 Control SD or SE Control sample size Control mean at (list value) at end on test period end of test period Baseline CAL:7.50mm 0.84 9.11mm 0.22 96 total patients to start SE 8.79 mm Control sample size Control mean Baseline Baseline Control SD or SE (list value) at end of test period 7.80mm 0.82 8.09 0.21 24 patients 10.75mm 0.29 SD or SE? Mean gain CONTROL (baseline-final) Mean SD (or SE) gain, CONTROL Caries data ANOVA used to test comparability of groups if homoscedasticity of residual variances; data at baseline used as regressor if relationship observed if homogeneity of slopes; data not fit for ANOVA tested with X2; Type I error at 5% SE 9.21 0.34 Statstical analysis notes Guentsch 2008 CAL To nearest mm at 6 sites per tooth 12 months 3, 6, 12 months full mouth not noted how many 37 (people) 5.64 0.91 36 3.9 0.86 SD 1.59 0.61 28 5.15 0.55 21 3.61 0.57 SD 1.48 0.53 N/A Calibration, reliability, power calculations; normal distributions verified with clinical data. ANOVA repeated measures - dropped out those who did not respond - appears that baseline data only included those who finished Lindhe 1983 CAL 1/2 mouth 50 weeks 10, 20 30 50 weeks 1/2 mouth 7 7.1 0.5 7 nr nr se 1.7 0.3 7 7.4 0.5 7 nr nr se 1.4 0.3 no t-test on difference, anova Ng, 1998 CAL 6 months 1 site per treatment combination 3 weeks, 6 weeks, 12 (many arms in trial); all teeth weeks, 24 weeks matched according to tooth type in both groups Ramberg, 2001 PAL with stents 1 year 3, 5 and 13 year data Supportive periodontal therapy 3is available in graph 4X/yr, for 13 years only July 2015 8 35people CAL:7.8 CAL:1.2 8 28 people at the end of 13 years. not reported not reported Sample size at 1 year not reported CAL:7.4 CAL:0.5 SD Not reported Not reported 8 CAL:9.0 CAL:1.9 8 CAL:9.9 CAL:0.8 SD Not reported Not reported Not reported experimental unit was the subject in all statistical analyses. These data were adjusted by Duncan's multiple range test for multiple comparisons. Within each treatment group, data from baseline and 24 weeks were compared and analyzed by Student t-test. The data of the with-scaling and without-scaling were also compared and analyzed by Student t-test. Statistical significance was accepted at probability level of ≤0.05 for all tests. not reported not reported SD 0.47mm ±0.6mm (SD) 80people not reported not reported 61 people people at the end of 13 years. Sample size at 1 year not reported not reported not reported SD 0.16mm ±0.5mm (SD) N/A No description Page 147 Systemic antimicrobials: tetracyclines + SRP - Updated literature Citation: Author, Year Outcome measure Tsalikis, 2014 CAL July 2015 Time period for No. Sites treated data presented in per mouth / No. this abstraction (as sites averaged per close to 9 months tooth as possible) 6 months not stated Test sample size Baseline Test mean Baseline 35 3.73 Test Test SD or Mean Mean SD Test SD or SE sample Test mean at SE (list difference (or SE) (list value) size at end end of test value) at SD or SE? TEST (final- difference, Baseline of test period end of test baseline) TEST period period 0.88 31 3.02 0.67 SD NOT REPORTED NOT REPORTED Control sample size Baseline Control mean Baseline Control SD or SE (list value) Baseline 35 3.77 1.24 Control Control SD Control sample or SE (list mean at end size at end value) at SD or SE? of test on test end of test period period period 35 2.87 0.89 SD Mean difference CONTROL (finalbaseline) Mean SD (or SE) Caries data difference, CONTROL NOT REPORTED NOT REPORTED not reported Statstical analysis notes None Page 148 Systemic Antimicrobials- Review authors’ judgment of each risk of bias item as low, unclear, or high for each included study Selection bias Domain: Random sequence generation Citation: Author, Year Support for judgment Review author's judgment Performance bias Allocation concealment Support for judgment Review author's judgment Masking of participants Support for judgment Review author's judgment Masking of personnel Support for judgment Were the groups treated the same except for the intervention? Detection bias Attrition bias Reporting bias Masking of outcomes assessment Incomplete outcome data (Include percent lost to follow-up) ≤10% low; 11-20% unclear; >20% high Selective reporting Support for judgment Review author's judgment Support for judgment Review author's judgment Support for judgment Review author's judgment "if indicated OHI" High no mention of examiner blinding High none lost Low Review author's judgment Support for judgment Review author's judgment AMX/MET Berglundh, 1998 randomly created two different samples assigned sample to tx Cionca 2009 Computer generated table Flemmig 1998 NR High Not described unclear high Low The treatment group was concealed from the patient, clinical examiner (NC), therapist (GU), and statistician (AM) Low No differences noted Low The treatment group was concealed from the patient, clinical examiner (NC), therapist (GU), and statistician (AM) Low 47/51 completed (8% lost) Low unclear no mention of masking or blinding of examiner or therapist unclear yes low no mention of examiner blinding unclear 10 out of 48 high Low Low neutral package containing the test or placebo medication, identical in appearance and only marked with the subject number Low The treatment group was concealed from the patient, clinical examiner (NC), therapist (GU), and statistician (AM) unclear alternate assignment high NR no evidence of clinical trial registration no mention of masking or blinding of examiner or therapist blind, placebo used Goodson, 2012 "randomly" & "computer generated number" Low Not described Unclear not placebo controlled high therapist and statistician were given treatment identity. high oral hygiene reinforced if necessary all sites with PPD >=5 mm and BOP SRP high Designated clinical examiners were blinded low 44 out of 231 or 19% Unclear Mombelli 2005 not clearly randomized High Not specifically addressed Unclear Placebo capsules and gels Low not clearly stated Unclear Yes Low Implied but no stated Unclear 2/16 lost (12.5%) Unclear Low allocation distributed in numbered opaque envelopes, not known by randomizer Unclear Riberiro, 2009 randomized using computer generated list Low randomization not broken until all data collected Low allocation not known until all data collected Low all patients given SRP and either placebo or antibiotics Unclear PAL reported but not PD or other perio measures Low blinded, and duplicate measurements Low 3/28 excluded because they did not show up to all appointments No bias detected Low none low Clinical trial registration > 5 years after trial completion No a priori hypotheses no evidence of clinical trial registrationNo a priori hypotheses reported on primary&second ary outcomes HIgh Unclear Low Metronidazole Palmer et al. Brit Random Number Dent J Table 1998;184:548-552. Low no details Unclear Not reported; no placebo pills High Not reported High all patients given SRP Low Single blind and randomized Low 93% (84/90) pts completed the 6 month study Low Reported primary and secondary outcomes, but no baseline data for CAL Unclear Palmer et al. JCP 1999;26:158-163. Random Number Table inferred from Palmer 1998 Low no details Unclear Not reported; no placebo pills High Not reported High all patients given SRP Low Single blind and randomized Low 93% (84/90) pts completed the 6 month study Low Reported primary and secondary outcomes, but no baseline data for CAL Unclear Haffajee 2007 Random number table Low Assignment by clinic cocoordinator Unclear No - single blinded High No-single blinded High Yes Low Masked Low 67/98 subjects had complete data for all monitoring visits; ITT used with LOCF; if only baseline data, subject excluded High AL at 12 months for pockets with baseline PD>6 mm primary outcome measure Low Gomi 2007 No details given Unclear No details given Unclear No details but likely not High No details but likely not TBI based on need Unclear Not stated Unclear No losses Low No bias detected Low Masked Low 67/98 subjects had complete data for all monitoring visits; ITT used with LOCF; if only baseline data, subject excluded High AL at 12 months for pockets with baseline PD>6 mm primary outcome measure Low Azithromycin High Haffajee 2007 Random number table Low Assignment by clinic cocoordinator Unclear No - single blinded High No-single blinded High Yes Low Mascarenhas 2005 Selecton from a bag Low Masked, but no details given Unclear Not masked High Randomized after SRP Low Yes Low Examiner masked Low 3.2% loss Low No bias detected Low Oteo 2010 computergenerated randomization list Low Identical pills, treatment assignment not revealed until after study Low treatment assignment not revealed until after study low 2/14 lost in placebo (14%); 0 in test group; 2/29 total (7%) Low No bias detected Low Low All examiners and study personnel masked Low Examiners and statisticians had no knowledge of assignments Low 2/40 lost = 5% Low No bias detected Low High Mentions double masking so this is the only possiblity although not described Low No losses Low Data interpolated off graph and not provided explicitly Unclear Low Assessor masked Low 3/40 lost 7.5% Low No bias detected Low High in control group subjects who did not resond were dropped out for retreatment-thus under reporting more severe subjects no intent to treat data shows up in baseline data where these retreat subjects were not included High Low No bias detected, but did not report the distribution of the pockets in the study - which could inlfuence values. Unclear High Sampaio 2011 computergenerated table Low Sealed envelopes Low Opaque plastic bottles; code breaking after statistical analysis Low Identical pills Low Capsules identical Unclear Unclear how this can be double masked? Low Yes Low Yes No differences noted Low Yashima 2009 No details given Unclear No details given Unclear Masking not possible; no placebo described yet stated "double blind" Pradeep 2011 computer generated list prepared by an independent study coordinator Low No details given Unclear Yes Unclear No placebo, patients taking antibiotics "extensively informed about the intake of the prescribed medication" Unclear Double-blind but no other details; All capsules of identical appearance High No High Since no placebo, only tx groups received extensive info about the medications Unclear Unclear double blinded but no details Unclear Yes Low SRP performed before treatment group assignment, examiners all blinded to treatment group Low all patients given SRP and either a dose or placebo Unclear No - different SRP methods Clarithromycin Low Yes Moxifloxacin Guentsch 2008 randomization performed independently at each center, no other details Unclear No details given, but control patients not given placebo High No High Since no placebo, only tx groups received extensive info about the medications Unclear Yes; masked to tx allocation Low 7/28 in control and 1/37 in treatment dropped out (in control group, patients who needed further treatment were dropped) Low Double blind; assume clinician but not explicitly stated Unclear 79/96 pts completed study (18% lost) Yes; masked to tx allocation Low 7/28 in control and 1/37 in treatment dropped out (in control group, patients who needed further treatment were dropped) High in control group subjects who did not resond were dropped out for retreatment-thus under reporting more severe subjects no intent to treat data shows up in baseline data where these retreat subjects were not included Low double blinded but no details Unclear no info. Assumed no loss Unclear No bias detected Low Low examiners all blinded to treatment group assignment Low reported on primary&second ary outcomes Low high only subjects who remained in the study the entire 13 year period were included in the analysis; selected different teeth in each did not discuss how selected High Tetracyclines Al-Jaburi 1989 Unclear No details given Guentsch 2008 randomization performed independently at each center, no other details Unclear No details given, but control patients not given placebo Unclear No placebo, patients taking antibiotics "extensively informed about the intake of the prescribed medication" Lindhe 1983 randomized but no method reported unclear not reported Unclear double blinded but no details Ng,1998 Ramberg, 2001 July 2015 Described as random but details not given randomized but no details no details Unclear Unclear No details provided no details Unclear Unclear patients given placebo if not an intervention no placebo Unclear High Double blind but no details no details regarding masking Unclear High Yes; no other differences described yes low no details Low High none lost 7/35 in test group and 19/80 in control group lost to follow up. Greater than 20% loss to follow-up Page 149 Systemic Antimicrobials- Updated literature Selection bias Domain: Random sequence generation Performance bias Allocation concealment Masking of participants Masking of personnel Were the groups treated the same except for the intervention? Detection bias Attrition bias Reporting bias Masking of outcomes assessment Incomplete outcome data (Include percent lost to follow-up) ≤10% low; 11-20% unclear; >20% high Selective reporting Citation: Author, Year Support for judgment Review author's judgment Support for judgment Review author's judgment Support for judgment Review author's judgment Support for judgment Review author's judgment Support for judgment Review author's judgment Support for judgment Review author's judgment Support for judgment Review author's judgment Support for judgment Review author's judgment Miranda, 2014 AMX/MET computer genrated Table low not reported unclear Placebo Pill low Clinician was blinded low yes. All received OHI. low evaluator was blinded low up to 15% LOSS TO FOLLOWUP low reported all data low low Codebook manager kept all patient and allocation data througout the study, securing masking of the clinical research staff low Codebook manager kept all patient and allocation data througout the study, securing masking of the clinical research staff low Codebook manager kept all patient and allocation data througout the study, securing masking of the clinical research staff low up to 2% LOSS TO FOLLOWUP low reported all data low low low evaluator was blinded low 22% loss to follow-up high all data reported low low evaluator was blinded low 0 loss to followup low all data reported low low Examiners were not aware of the tx low 12% Loss in tx group. No loss in control unclear reported all data low Preus, 2013 Metronidazole Computer generated sequence Han, 2012 Azithromycin computergenerated randomization list Martande, 2014 Azithromycin computer genrated Tsalikis, 2014 Tetracyclines Randomization software July 2015 low Placebo Pill low randomization list kept by one of the oughtus utill all tx and evaluatin were completed low pts masked. Placebo and tx pill had exact same appearance low clinician, evaluater and biostatistician were blinded low not reported unclear pts masked. Placebo and tx pill had exact same appearance low Clinian was blinded low low "randomization list was kept by one of the authors until patients were eligible for the study low Upon completion of the tx (SRP), subjects were allocated to two groups according to the randomization list low unclear Placebo Pill low yes. All received OHI. all received OHI and supragingival plaque and calculus removal at eval visits Yes. All received OHI and re SRP at all evaluation visits Yes, all receivewd OHI Page 150 Chlorhexidine chip +SRP - Study characteristics Citation: Author, Year Azmak 2002 DIMITRA, 2010 Gonzalez 2011 HEASMAN, 2001 Paolantonio, J Perio 2008a (all data) Paolantonio et al. J Biol Regulat Homeostatic Agents, 2008b July 2015 Special population? (e.g. smokers) and other data regarding inclusion exclusion criteria for patients and teeth Severity of disease (e.g. refractory, mild, moderate) Turkey No Moderate to severe chronic periodontitis (68mm) Greece Inclusion: CAL ≥5mm; 4 teeth with pocket depth ≥5 and ≤ 7mm; at least 20 teeth; no antibiotics for previous 3 months; no allergies to antibiotics; no perio tmt previous 12 months systemic pathogies; Exclusion: no pregnant or lactating women. Smoking status recorded. Germany Inclusion: Systemically healthy individuals aged >35 and <65 years w ere enrolled if they had ≥12 teeth w ith PD ≥5 mm w ith BOP. Exclusion criteria w ere: 1) systemic antibiotic therapy w ithin the past 6 months, 2) hx of allergy to CHX, 3) perio tmt w ithin the past 6 months, 4) pregnancy, and 5) heavy smokers (>10 cigarettes per day). UK Recall (maintenance) patients. Inclusion criteria: signed informed consent; min of 10 natural uncrow ned teeth; a min of 1 pocket/quadrant w ith a pocket probing depth (PPD) of at least 5 mm w ith persistent BOP; nonsurgical phase of therapy completed at least 3 months prior to bas line. Exclusion criteria: early onset periodontitis; any teeth w ith furcation involvement; systemic antimicrobial therapy w ithin 2 months prior to entry; hx of allergy to CHX; smoking; hx of perio surgery; perio tmt less than 3 months prior to the baseline visit. Italy Non-smokers, at least two teeth with PD≥5mm. Exclusion criteria: smoking, pregnancy, allergy to CHX, systemic antimicrobial and anti-inflammatory drug therapy within 3 months prior Moderate to advanced to the study, periodontal perio (at least two sites treatment undertaken <6 5 mm or greater) months prior to the preliminary visit, or the presence of a removable prosthesis; furcation sites and teeth with a fixed prosthesis. Country Test subject age Control subject (mean, median, age (mean, range as median, range as described) described) 36-62 36-62 Control Dose/ duration/ frequency/ timing if applicable Test (typically adjunct) SRP of entire mouth. One isolated mesial interproximal pocket with probing depth of 6-8mm and BOP served as the control site n/a CHX chip + SRP Chiorhexidine chip (2.5mg chlorhexidine gluconate in gelatin) was placed in four pre-selected pockets with Periochip® probing depth and probing CHX + SRP attachment level ≥ 5 mm and ≤ 7mm with a blunt instrument by the same clinician who performed SRP. Generalized Chronic periodontitis Mean = 46.35, Mean = 48.75 ±7.31 (range 36-64 ±10.15± (range 37years) 75 years) SRP Full mouth SRP w as performed in tw o quadrants under local anesthesia, in tw o sequential visits, one day apart / Mechanical instrumentation included ultrasonic instrumentation follow ed by hand instruments (Gracey curettes SG 3/4, 11/12, 13/14, Hu-Friedy, Chicago, IL, U.S.A.). / Duration of instrumentation for each visit ranged betw een five and 10 minutes per tooth. Chronic periodontitis Specific age data are Specific age data are not provided; just not provided; just stated individuals stated individuals w ere w ere aged >35 and aged >35 and <65 <65 years; 24 years; 24 recruited, recruited, equal # of equal # of men and men and w omen; w omen; equal-->12 equal-->12 per group per group w ith equal # w ith equal # of men of men and w omen. and w omen. SRP plus placebo chip Prophy plus CHX or Placebo chip for 10 days. Then SRP plus CHX or Placebo chip again. 12 sites with ≥ 5mm were selected for the intervention sites Periochip® 2.5 mg CHX gluconate SRP Supragingival ultrasonic scaling and a prophylaxis of all teeth w ere performed and all target sites w ere root planed under local anaesthesia for a maximum of 5 min for each tooth. Target sites w ere randomised at split mouth level (left side/right side) to one of the 2 treatment groups; SRP alone, SRP + PerioChip. Follow ing debridement, target sites w ere irrigated gently w ith cold saline and then left for 10 min to achieve haemostasis prior to placement of PerioChips. SRP + PerioChip Moderate to severe chronic periodontitis 42.6 (sd 12.6) Range 34-59 years aged 33-65 SRP only SRP at baseline visit under local anesthesia for entire mouth; 2nd session within 48 hours. Each SRP + appt=2hrs; hand PerioChip at instrum-Gracey's. one site SRP performed carefully for 5 minutes at the experimental sites. SRP only Screening visit 2 wks prior to baseline appt. During screening visit all pts. received supragingival scaling, OHI and PD measurements at 4 sites around each tooth (Mesial-, Midand Disto-Buccal and Mid-Lingual/Palatal). Control Tx consisted of SRP only using Gracey curettes. 82 systemically healthy pts. (49 females & 33 males). Inclusion criteria: minimum of 10 natural teeth and periodontal disease characterized by 2 or more natural teeth with PD ≥ 5 Italy Mean age not Mean age not mm and BOP. Exclusion Moderate and Advanced reported. Range reported. Range criteria: smoking, Chronic Periodontitis for all pts. Was 31- for all pts. Was 31pregnancy, allergy to 63 yrs. 63 yrs. CHX, systemic antimicrobial or antiinflammatory drug therapy within 3 mo. prior to study, and periodontal Tx undertaken < 6 mo. prior to baseline visit. Test (typically adjunct) Was standard Dose/ duration/ frequency/ Trial design (split counseling mentioned timing including mouth / parallel as part of control simultaneous/ before/ group / cross over) treatment? after Control SRP (of entire mouth) + CHX chip placed in one isolated mesial interproximal pocket with probing dept of 6-8mm and BOP Duration of study Adverse events reported Yes split mouth 6 months Did not seek or report adverse events All patients received detailed oral hygiene instructions and interdental toothbrushes w ere providedw ith identical nylon, soft, multitufted TB and fluoride toothpaste. TBs w ere replaced every3 months. Subjects instructed not to use an anticeptic mouthw ash throughout the study. Parallel group 6 months No adverse effects resulting from CHX placement, such as discomfort, pain or swelling, as reported by patients, were recorded. 6 months Not reported Prophy plus CHX or Placebo Parallel group chip for 10 days. Then SRP Yes, repeated Randomized, doubleplus CHX or Placebo chip instructions in OHI were masked, placeboagain. 12 sites with ≥ 5mm given. controlled clinical were selected for the trial intervention sites PerioChip (2.5mg chlorhexidine gluconate in gelatin) placed at baseline Not reported Split mouth 6 months Yes, 1 person reported nontreatment aphthae of buccal mucosa CHX at time of SRP; Yes, OHI given. No flossing after placement of CHIP for 10 days; no chemotherapuetic mouthrinses or oral irrigation during study. At 3-month visit, supragingival scaling + OHI split mouth 6 months Did not seek or report adverse events OHI at screening visit Split mouth 6 months Not Reported SRP + CHX 1 CHX chip per test site at chip time of SRP Page 151 Chlorhexidine chip + SRP - Outcomes Data Citation: Author, Year Azmak 2002 Outcome measure CAL DIMITRA, 2010 Probing Attachment Level (PAL) Gonzalez 2011 Main outcome variable was the change over time of the microbiologic data within both groups. CAL change over time was a secondary outcome. HEASMAN, 2001 Time period for data presented in this abstraction 6 months Other time periods for which data are available No. Sites treated per mouth / No. sites averaged per tooth Test sample size Baseline 1 and 3 months Two interproximal sites (one tx and one control) from mesial surfaces of anterior teeth that were 6-8mm. So two sites per mouth, and one site (mesial) per tooth. 22 individuals, one pocket per individual 6 months Baseline, 3 and 6 months Clinical data measured for all teeth in dentition, although chip only at 4 pockets. / PAL measured at 6 sites per tooth / 6 months Day 0, prior to Chip placement on day 14, 10 days prior to SRP; 1, 3 & 6 months 12 teeth w ith the deepest periodontal pockets (PD ≥5 mm) w ere selected (test teeth) for one of the tw o treatments: CHX or placebo chips. If more than one site w ith PD ≥5 mm w as present in a single tooth, a maximum of tw o chips per tooth w ere inserted. 144 total teeth per group 27 12 Test mean Baseline 8.83 6.47 Test SD or SE (list value) Baseline 1.34 SD ±0.85 Not reported; Not reported; graphic display of graphic display Box plots with of Box plots with percentiles percentiles Estimate 4.9 Test sample size at end of test period Test mean at end of test period 20 Figure 2 presents improvement of CAL in graph, no raw data. Improvement estimated at 1.75 mm, but this is eye-balling it 25 12 4.5-5.5 (25th and 75th percentiles) 5.07 Test SD or SE (list value) at end of test period SD or SE? Unclear Not stated; assumed SE for difference ±1.05 Not reported; graphic Not reported; display of Box graphic display of plots with Box plots with percentiles percentiles Estimate 3.6 SD not reported 4.5-4.5 (25th and 75th percentiles) Mean gain TEST (Baselinefinal) Mean SD (or Control SE) gain, sample size TEST Baseline Figure 2 presents improvement of CAL in graph, 22 individuals, no raw data. Estimate: 0.2 one pocket per Improvement individual estimated at 1.7 mm, but this is eyeballing it Not reported Not reported Data text indicates presented in 1.17mm gain figure 2 only. in attachment Raw data not reported 29 12 Control mean Baseline 8.72 6.38 Control SD or Control SD or Control sample Control mean at SE (list value) SE (list value) size at end on test end of test period at end of test Baseline period period 0.84 1 Not reported; Not reported; graphic graphic display display of of Box plots Box plots with percentiles with percentiles 4.2-6.1 (25th and 75th Estmate=5.0 percentiles) 20 25 12 Figure 2 presents improvement of CAL in graph, no raw data. Estimate: 0.2 Improvement estimated at 1.6 mm, but this is eyeballing it 4.98 Not reported; graphic display of Box plots with percentiles Estmate=4.5 ±1.37 Not reported; graphic display of Box plots with percentiles SD or SE? Mean gain CONTROL (baseline-final) Mean SD (or SE) gain, CONTROL Caries data Statstical analysis notes Not stated; assumed SE for difference Figure 2 presents improvement of CAL in graph, no raw data. Improvement estimated at 1.70 mm, but this is eye-balling it Unclear not reported Changes in CAL are based on visual estimates based on figure 2 NA The subject (not the site) was chosen as the observational statistical unit, and a probing depth (PD) difference of 2 mm was chosen as a clinically desirable primary outcome. To detect differences with a signif level (alpha) of 0.05 (two tailed), power of 80% (type beta) and an expected standard deviation of the afterbefore differences =2, a sample size of at least 25 in each group is required. To check differences between groups across all time points the general linear model, repeated measures procedure was appliped with the patient as the observational unit. ANOVA was also implemented at each time point. NA Because the parameters of interest were not normally distributed, the median, quartiles, maximum, and minimum were used for description of data distribution. Summarizing data of discrete variables and relative frequencies were computed separately by group. Because normal distribution could not be assessed, the change over time within groups and among the treatments was analyzed by means of the Wilcoxon-Mann-Whitney test. Results are shown in the form of tables and box plots. 144 test teeth in each group. Data were analysed on an intention-to- treat basis with the subject as the unit of statistical analysis. The protocol-defined primary outcome variable was the reduction of PPD from baseline. At the 1, 3 and 6 month visits, the change from baseline for PPD, BI and CAL for each site was calculated. A mean value was calculated for each treatment (split- mouth) and an overall mean of the dif- ferences from baseline was calculated for each treatment. Summary statistics were determined and 2 sample t-tests undertaken to identify statistically sig- nificant differences between the treat- ments (at p=0.05). In addition, the pro- portion of sites within a patient showing an improvement from baseline of PPD of >1 and >2 mm were recorded. The distribution of the scores at the 6 month visit was compared across the treatments using nonparametric, Cochran-MantelHaenszel row means test. SD not reported 3.5-5.0 (25th and 75th percentiles) Not reported text indicates 0.79 mm gain in attachment Not reported not reported CAL 6 months 24 subjects; 135 PerioChips were placed (87 at molar 26 (served as 1, 3 and 6 months sites) and 165 control sites their own control) (102 molar sites) were identified 14.23 SE, 0.19 24 Not reported Not reported SE gain, 0.43 0.15 26 (served as their own control) 14.14 0.16 24 Not reported Not reported SE 0.15 0.09 NA RAL (Relative Attachment Level), all data 6 months 2 interproximal sites (one tx and one control) not reported n/a unclear. Assumed to be 116 Not reported Not reported SE changes in RAL for all sites at 6 months approximately 1.1mm 0.1 116 not reported N/A unclear. Assumed to be 116 Not reported Not reported SE Figure 4a presents changes in RAL for all sites estimated to be 0.6mm 0.1 not reported Figure 4b presents changes in RAL for sites 7mm or greater at 6 months approximately 1.2mm 0.2 Paolantonio, J Perio 2008a (all data) 116 1, 3, 6 months RAL (Relative Attachment Level) , 7 mm or greater data Paolantonio et al. J Biol CAL, PD, BOP and Regulat GCF Acid Phosphotase Homeostatic Levels Agents, 2008b July 2015 2 interproximal sites (one tx and one control) 6 months 6 months Baseline and 6 months 116 not reported 2 test teeth per mouth. 82 pts. X 2 sites Number of sites per tooth not (2 teeth) = 164 8.7 ± 1.4 (mm) reported. test sites n/a unclear. Assumed to be 116 Not reported Not reported SE ± 1.4 mm 82 patients, 164 Test Sites 7.3 ± 0.54 (mm) ± 0.54 mm Not reported 116 Not reported, 82 pts. X 2 Estimated from but estimated sites (2 teeth) Figure: 1.1 from = 164 test figure:0.1mm sites not reported 8.8 ± 2.1 (mm) Table 2 has data on % gain in RAL from baseline categorized into greater than or equal to 2mm, 1mm, or less than or equal to 0mm.. Abstract states that changes in RAL betw een control and tx group w as 0.64mm at 6 months (p<0.001) Parametric methods w ere used w hen appropriate after havingtested the normality of the data, using the Shapiro-Wilk test and Q-Q normality plots; the equality of variance among the data sets also w as tested using the Levene test and Q-Q normality plots of the residuals. A multivariate three-w ay analysis of variance (three-w ay MANOVA) w as performed to assess the differences in PD and RAL. The three factors w ere time and treatment (as repeated factors) and investigator/study site (as an independent factor). A paired-sample t test w as used, w hen appropriate, for pairw ise comparisons. N/A unclear. Assumed to be 116 Not reported Not reported SE Figure 4b presents changes in RAL for sites 7mm or greater at 6 months approximately 0.7mm 0.1 not reported ± 2.1 mm 82 patients, 164 Sites 7.9 ± 1.41 (mm) ± 1.41 mm Not reported Not reported, but estimated from figure: 0.5 mm Estimated from Figure: 0.1 Not Reported An additional analysis included calculation of the clustered PD and RAL changes at 3 and 6 months w ith respect to the baseline scores. These clustered changes w ere set as ≤0 (no reduction/gain), 1, and ≥2 mm. These clusters w ere considered ordinal data, and the corresponding significances of the differences betw een the treatments (for each of the 3- and6-months time-point changes) w ere evaluated w ith the Wilcoxon test. Parametric methods use when appropriate after having tested normality of data by means of a Shapiro-Wilk test and by Q-Q normality plots. Changes in clinical parameters between baseline and 6 months were analyzed within Tx groups using Student's t-test for paired samples. The same analysis was used to determine significant difference bgetwee test and control groups a baseline and 6 months. Bonferroni corrections were applied to adjust p values in the pairwise comparisons. Page 152 Chlorhexidine chip + SRP - Review authors’ judgment of each risk of bias item as low, unclear, or high for each included study Random sequence generation Citation: Author, Year Azmak 2002 Support for judgment flip of a coin Review author's judgment Allocation concealment Support for judgment low Not reported Review author's judgment Masking of participants Support for judgment Masking of personnel Review author's judgment Support for judgment Unclear Blinding claimed, but not described in detail. It is likely that personnel were aware of the site they were placing chip in High unclear Blinding claimed, but not described in detail. Unclear only the examiner was described as blinded High only the examiner was described as blinded Review author's judgment Unclear Were the groups treated the same except for the intervention? Masking of outcomes assessment Review author's judgment Support for judgment low Blinding claimed, but not described in detail. It is unclear if person conducting evaluation was the same as the person placing the chip Yes Support for judgment both groups were treated similarly Review author's judgment Incomplete outcome data (Include percent lost to follow-up) ≤10% low; 11-20% unclear; >20% high Support for judgment Selective reporting Review author's judgment Support for judgment Review author's judgment Unclear Unclear 2 drop outs due to use of antibiotics Low all data was reported, but in graphs rather than raw data; limited information provided on clinical parameters Low Outcome measurements by blinded examiner Low 6 of 56 lost (11%); although half intentionally excluded because nonoptimum OHI unclear No bias detected Low DIMITRA, 2010 Random tables Low Kept by 1 investigator until patients were eligible for study GONZALES, 2011 Number assigned in sequence of their presentation; randomly allocated-doesn't say how; Unclear Not described Unclear Not described; just said study was double masked Unclear 1 person examined and was blinded Low Yes Low Outcome measurements by blinded examiner Low Not reported; assumption is 0 unclear No bias detected outside of not describing/rep orting previous areas. Unclear HEASMAN, 2001 Target sites were randomized at split mouth level Unclear Not described Unclear Not described Unclear 2 calibrated examiners Unclear Yes Low Periochip placed by clinician when examiners not present Low 2 of 26 lost Low No bias detected Low Paolantonio, 2008a coin toss low Not reported unclear not described Unclear Not reported Unclear yes low Examiner was masked Low None reported; assumption is 0 Unclear Raw data, SD and loss to follow-up not reported high High Intervention (but not control group) told not to use dental floss for 10 days after placement of chlorhexidine chip. high Examiner unaware of attribution of sites Low No losses Low Mistakes in reporting High Paolantonio 2008b No details given July 2015 Unclear No details given Unclear Not blinded (single blind) High Not blinded (single blind) Page 153 Locally-delivered antimicrobials: doxycycline hyclate gel + SRP - Study characteristics Citation: Author, Year Country Special population? (e.g. smokers) and other data regarding inclusion - exclusion criteria for patients and teeth Severity of disease (e.g. refractory, mild, moderate) Control Test subject subject age age (mean, (mean, median, median, range as range as described) described) Control Control Dose/ duration/ frequency/ timing if applicable Test (typically adjunct) Test (typically adjunct) Dose/ duration/ frequency/ timing including simultaneous/ before/ after Agan 2006 Turkey 2 or more tooth sites with PD >= 6mm and CAL >= 3mm and BOP. No antimicrobial therapy within 6 months of BL. Study also included AgP but subjects reported separately from CP. Advanced chronic periodontitis Mean 55 Same as years, range test: Split41-49. SD not mouth given design SRP SRP under local anesthesia with hand instruments, limited to 10 min per tooth / Single visit doxycycline hyclate+SRP Single subgingival application of doxycycline hyclate 10% gel, covered with dressing for 1 week. / Total dose not specified. Gel to fill pocket. OHI given to all subjects Split-mouth 6 months None reported by subjects (mentioned in discussion) Machion 2004 Brazil SMOKERS. 1) patients diagnosed w ith chronic periodontitis, show ing a minimum of four periodontal pockets (probing depth [PD] ‡5 mm and bleeding on probing [BOP]) located on anterior teeth (canines and incisors); 2) patients w ho smoked ‡10 cigarettes/day for a minimum of 5 years; Exclusion: Exclusion criteria w ere as follow s: 1) patients w ho received scaling and root planing w ithin a period of 6 months prior to the study; 2) patients show ing the presence of periapical or pulpal alterations on qualifying teeth; 3) patients w ith a compromised heart condition or any systemic disorder that w ould require prophylactic antibiotic cover; 4) patients w ith a history of allergy to doxycycline hyclate or other tetracycline; 5) patients w ho used systemic or subgingival antimicrobials w ithin a period of 6 months prior to baseline examination; and 5) patients undergoing drug therapy that could affect the clinical features of periodontitis or the response to periodontal treatment. Chronic periodontitis 40.45+/-4.47 42.00+/-4.38 SRP plus saline irrigation SRP followed by irrigation with saline / instrumentation continued until root smooth and clean SRP+local doxycycline (Atridox, CollaGenx, Ft. Collins, CO) 10% No Parallel 6 months No adverse reactions from any of the patients in test group Machion, 2006 same as above same as above same as above same as above same as above same as above same as above same as above same as above 2 years no 12 months Not described same as above same as above Was standard Trial design counseling (split mouth Duration of mentioned as part / parallel study of control group / treatment? cross over) Adverse events reported Patients were enrolled for initial Patients with Type I Diabetes Mellitus Martorelli 2004 Brazil No other systemic disease / no antimicrobial tx in past 6 months / Each patient contributed two sites which were used for measurements. Sites included were required to have (1) probing depth (PD) X5.0mm and (2) bleeding and/or suppuration on probing Advanced periodontal disease Subjects age 35-55 years therapy which Scaling and root planing plus experimental doxycycline included oral SRP + placebo gel hyclate gel (methocel hygiene instruction, (Farmacia Escola Comunitaria supragingival "Patients seen at 6 Split mouth, (FAQFAR), University of ultrasonic weeks, 3, 6, 9, and Gel placed immediately ItaJai, Brazil) w ith 10% Gel placed immediately after SRP procedures 2 sites per instrumentation and 12 months for after SRP procedures doxycycline hyclate (MASE person appointments for fullperiodontal Produtos Quirnicos e mouth scaling and maintenance" which Farmaceuticos Ltda., root planning under was not described Cambuci, Sao Paulo, Brazil) test group) anesthesia prior to the experimental phase. July 2015 Page 154 Locally-delivered antimicrobials: doxycycline hyclate gel + SRP - Outcomes data No. Sites treated Time period for Other time per mouth / No. data presented in periods for which sites averaged per this abstraction data are available tooth Citation: Author, Year Outcome measure Agan 2006 CAL 6 months RAL gain measured at six sites per tooth, mm All pockets 6 months Machion 2004 7 days, 1 and 3 months presumably 2 sites in each group (one site each of test and control) in each of 10 subjects mean of 7.3 sites per patient, total of 314 sites, six sites per tooth Test sample size Baseline Test mean Baseline Test SD or Test sample SE (list size at end value) of test Baseline period Test mean at end of test period Test SD or SE (list value) at end of test period SD or SE? Mean gain Mean SD Control TEST (or SE) sample size (Baselinegain, TEST Baseline final) Control mean Baseline Control Control Control SD or SE sample Mean gain Mean SD Control SD or mean at (list size at CONTROL (or SE) SE (list value) end of value) at SD or SE? end on (baselinegain, Baseline test end of test final) CONTROL period test period period Caries data Statstical analysis notes 8.27 1.14 (appears to be SD for sites) (N=20) 10 NR NR NR 1.56 NR 10 8.72 0.94 (appears to be SD for sites) (N=20) 10 NR NR NR 1.44 NR NR "Tests w ere subjected to each treatment protocol on a patient-level basis." Changes form BL w ere analyzed by the repeated measures analysis of covariance. Intergroup differences tested using Bonferroni-corrected Wilcoxon signed rank test and considered P < 0.01 as significant. 24 not reported not reported 22 1.63 0.93 SD 1.63 0.93 24 not reported not reported 21 1.04 0.71 SD 1.04 0.71 N/A Examiner calibrated 24 not reported not reported 22 1.37 1.02 SD 1.37 1.02 24 not reported not reported 21 1 0.75 SD 1 0.75 N/A Examiner calibrated 24 not reported not reported 22 2.54 1.27 SD 2.54 1.27 24 not reported not reported 21 1.29 0.95 SD 1.29 0.95 N/A Examiner calibrated n.a. n.a. 19 n.a. n.a. n.a. 1.31 0.95(SD) n.a. n.a. 16 n.a. n.a. n.a. 0.99 0.85(SD) 7.2 0.4 11 4 0.4 SE 6.6 0.5 11 5.6 0.6 SE Not reported Not reported 10 subjects although data presented for sites (N=20) 45 days, 3 months RAL gain, mm 5-6 mm pockets (moderate) 6 months RAL gain, mm deep pockets 6 months Machion, 2006 RAL gain measured at six sites per tooth, mm All pockets. (site specific data also available, but in graphic form) 12 month Martorelli 2004 CAL = the distance from CEJ (cemento enamel junction) to the base of the pocket [Each patient contributed two sites which were used for measurements. Sites included were required to have (1) probing depth (PD) =>5.0mm and (2) bleeding and/or suppuration on probing.] 9 months July 2015 same as above total of 236 sites, six sites per tooth Twenty-two paired periodontal defects 6 weeks, 6, and 12 =>5.0mm in the months maxillary incisor or canine areas of 11 patients 11 Not reported Not reported 11 N/A Page 155 Locally-delivered antimicrobials: doxycycline hyclate gel + SRP - Review authors’ judgment of each risk of bias item as low, unclear, or high for each included study Domain: Selection bias Random sequence generation Citation: Author, Year Agan 2006 July 2015 Support for judgment Mouth sides randomized by coin flip Review author's judgment Low Performance bias Allocation concealment Support for judgment Not specified Masking of participants Masking of personnel Detection bias Were the groups treated the same except for the intervention? Review author's judgment Support for judgment Review author's judgment Support for judgment Review author's judgment Support for judgment Review author's judgment Support for judgment Unclear Termed "Blind" by authors but probably for lab assessments only Unclear Not described Unclear Yes Low Not specified Review Support for author's judgment judgment Unclear Machion 2004 No details given Unclear No details given Unclear No Unclear All SRP done by same masked operator low Yes Low masked Low Martorelli, 2004 unclear n.d. unclear "patient blind" low "operator blind" low Yes low n.d. unclear n.d. Attrition bias Incomplete outcome data (Include percent Masking of outcomes lost to follow-up) assessment ≤10% low; 11-20% unclear; >20% high 0/10 lost 43/48 completed study (10% lost); sites of both groups excluded if >= 2 mm AL at any time point; 3 sites from control and 1 from test excluded out of 314 sites No losses noted Reporting bias Selective reporting Review author's judgment Support for judgment Review author's judgment Low No bias detected Low Low No bias detected Low low good data report low Page 156 Locally-delivered antimicrobials: minocycline microspheres + SRP - Study characteristics Citation: Author, Year Country Special population? Severity of disease (e.g. smokers) and other data regarding inclusion - (e.g. refractory, mild, exclusion criteria for patients and teeth moderate) Control Test subject subject age age (mean, (mean, median, median, range as range as described) described) Control Control Dose/ duration/ frequency/ timing if applicable No Henderson 2002 Skaleric 2004 at least two pairs of adjacent 6-9mm pockets; each pair located on adjacent teeth in an interproximal space, on opposite New Zealand sides of the mouth. Each study site was required to have at least 3mm loss of attachment. / no perio tx at least 6 months prior / no antibiotics for prior 3 months / other disease restrictions / 6/15 subjects were current smokers, 6-40 cigarettes/day over 15-30 years Slovenia Type I diabetics (poorly controlled) / 7 smokers in test group and 3 in control group but stated p=0.18 / HbA1c 7.5% and adult periodontitis, as detertmined by the presence of four teeth in at least 2 quadrants with 5 mm periodontal pockets, two of which had 6-9 mm pockets and BOP / no systemic antibiotics for prior 3 months / no antiinflammatories prior 1 month / several co-morbidity restrictions. No Type II diabetics Moderate to severe chronic periodontitis Adult periodontitis SRP with ultrasonic and hand mean age of 46.3 years(35instruments + OHI. 69 years range) Local anesthesia when needed 42 41.6 SRP No Van Dyke 2002 U.S. at least two teeth having one site each with pocket depth (PD) >=6mm and with prostaglandin E2 (PG E2) levels > 66.2ng/ml in gingival crevicular fluid. Moderate to severe periodontitis Not stated Not stated SRP Single 90-minute session Completion within 48 hours / The use of hand curettes, ultrasonic instruments and local anesthesia were permitted / a timed (20 minute) supragingival cleaning at 12 weeks for both groups For those patients receiving no Tx or SRP only irrigation of ,the surrounding test area was performed using sterile wvater or saline to ensure patient blinding. Test (typically adjunct) Test (typically adjunct) Dose/ duration/ frequency/ timing including simultaneous/ before/ after Was standard Trial design counseling (split mouth Duration of mentioned as part / parallel study of control group / treatment? cross over) OHI given to all pts following baseline SRP was followed by a single application of I exam / consisting of mg of minocycline in the form of Minocyctine SRP+Local tlie Periodontal Therapeutic System) (MPTS) into minocycline Bass toothbrushig one of the four sites selected at random by Split mouth microencapsulated methaod and another clinician, who also randomly selected microspheres (Arestin) interproitial cleaning one of thle two sites on the opposite side of with the use of floss the mouth to be design ated the Remote site. and/or interproximial brushes. SRP+Local Arestin (minocylcine microspheres) containing 1 mg minocycline SRP+ Local Arestin (minocylcine microspheres) containing 1 mg minocycline All pockets >= 5 mm at baseline after SRP and bleeding subsided / and at 12 weeks. 4 mg of dry powder - 1mg of minocycline and 3mg of polymer Treatment was applied to any, of six pockets >= 5mm in the two chosen study teeth. No No Adverse events reported 6 months Not assessed Parallel group 24 weeks Not described Parallel 6 months An SRP + MPTS patient experienced tw o adverse events (AEs) - a black hairy tongue ruled to be possibly dtug related by the investigator and abscess formation at study sites w ith oedema, w hich w as not considered to be related to study medication. There w ere three AEs reported in the no Tx group: toothache in a tooth adjacent to a study tooth, ulcerative stomatitis, and melena. They w ere not related to study medication. An SRP patient reported an event of myalgia, and SRP patient reported an event of a gramulomatous lesion, and an MPTS patient reported an event of toothache in a non-study tooth, and SRP + MPTS patient reported an event of rhinitis, and an MPTS patient reported an event of headache. Regarding the gingival margin, the SRP + MPTS group tended to display the! least redness and sw elling at 14 days and one month. The same trend w as obsetved at six months, but to a lesser degree There w as no significant difference in intensity and extent of tooth staining at any assessment point among the treatment groups. There -w ere no abnormal clinical chemistry or haemnatological results observed in the study for any of the groups. NDA 50-781: 103A USA At least 4 teeth with PD 6-9 mm and BOP; smokers not excluded Moderate to advanced 48.6 (10.1) 48.0 (10.0) SRP No details given NDA 50-781: 103B USA At least 4 teeth with PD 6-9 mm and BOP; smokers not excluded Moderate to advanced 49.6 (10.2) 47.4 (9.5) SRP No details given (Williams et al 2001) USA July 2015 30+ years of age, in good general health, no perio treatment within 6 months or antibiotics within 3 months, No medications that "could affect periodontal status or healing" moderate to advanced periodontitis (4+ teeth with pockets 6-9 mm) 47.7 years 49.1 years; SRP alone group scaling and root planing alone Once at baseline. Not time limit. No apparent maintenance therapy at 3 or 6 months (in either test or cotrol groups) SRP+Arestin (minocycline minospheres) SRP+Arestin (minocycline minospheres) No details given Not described Parallel group 9 months No details given Not described Parallel group 9 months minocycline microspheres inserted 1 mg minocycline in each pocket at baseline, in all pockets >= 5mm 3 and 6 months. At basleine, placed after at baseline. Product instrumentation. At 3 and 6 month visits, reapplied to test sites placed after probing. at 3 and 6 months. Not specified. Parallel arm 9 months Most common treatment emergent AEs: periodontities, ; stat sig difference in treatment emergent Aes w ith test vs control. Non drug-related, serious AEs w ere: body pain, tachycardia, myocardial infarction, colitis, pancreatitis, asthma, urinary incontinence, uterine disorder, accidental injury, carcinoma, hernia, viral infection, emolus, elective surgery, prostatic carcinoma. Adverse events were reported by 62.4% and 68.3% of patients in cntrol and combination therapy groups respectively. The incidence of these adverse events was similar among treatment groups. The most common adverse events are listed in Table 4, and included headache, dental infection, increased periodontitis, tooth sensitivity, tooth caries, dental pain, gingivitis, and stomatitis. No clinically significant changes in vital signs or oral hard or soft tissues were noted in these studies. Page 157 Locally-delivered antimicrobials: minocycline microspheres + SRP - Outcomes data Citation: Author, Year Outcome measure Time period for data presented in this abstraction Other time No. Sites treated per periods for which Test sample size mouth / No. sites data are Baseline averaged per tooth available 6 months 1 site per mouth tx with test; two control sites, one adjacent and one remote 15 Not reported Not reported Not reported 1 site per mouth tx with test; two control sites, one adjacent and one remote 15 Not reported Not reported Not reported all periodontal pockets 5' mm or deeper treated with minocycline; CAL measured at 6 sites per tooth 10 patients 6.89mm 1. 36 Not stated 3.8 six sites on two teeth selected for the study. The two teeth were selected based upon the presence in at least one of the sites (in each tooth) of A PD 6mm and GCF levels of PGE 2 > 66.2ng/ml. Although treatment was administ’d only at sites ≥5mm on the study teeth, clinical indices were monitored at all six sites. 12 11.01 not given 12 12 11.01 not given 12 11.01 Not described 121 5.56 Test mean Baseline Test SD or Test sample SE (list size at end value) of test Baseline period Test mean at end of test period Test SD or SE (list value) at end of test period SD or SE? Mean gain Mean SD TEST (or SE) (Baselinegain, TEST final) Control sample size Baseline Control mean Baseline Control SD or SE (list value) Baseline Control Control Control SD or SE sample mean at (list size at end of value) at end on test end of test period test period period SD or SE? CAL gain, mm Pocket depths were recorded using a manual probe with 1mm increments and wvith a tip diameter of 0.5mm. The measurements were made to the nearest Henderson millimetre. Sites were rounided down if 2002 the reading fell1 between two, millimetre markings. Frequency and percentage of CAL gain >/= 2 mm Skaleric 2004 3 months 6 months CAL attachment level (although states "loss" it is judged to be "level"), mm 24 weeks 6, 12, 18 weeks measurements at 6 sites per tooth excluding 3rd molars Not reported Not reported SD 2.1 1.5 6 months Change in CAL (baseline given), mm 6 months Van Dyke 2002 >=5 but <6 mm SITES Months 1 and 3 Change in CAL (baseline given), mm 6 months >=6 mm SITES NDA 50-781: 103A CAL at baseline, and CAL gain at 9 months NDA 50-781: 103B CAL at baseline, and CAL gain at 9 months (Williams et al 2001) CAL (paper's primary outcome was PD. BOP was a secondary outcome. CAL was listed as a safety outcome) July 2015 9 months 9 months 9 months None None Not described Treated all sites >= 5mm; PD 3 and 6 months measurements at 6 after randomization sites per tooth recorded in whole mm using round-down procedure 128 249 5.21 Caries data Statstical analysis notes With Only 15 patients, the power of this study is low and therefore clinical differences between sites would have to be proportionately greater to show Not statistical significance. It is therefore nor Adjacent reported surprising that for the majority of clinical Adjacent site, CAL mneasurements, statistiqaliv site, CAL gain: significant diffcrences betwveen treatments gain: (1.4) were not shown. This may be due to no truse 1.4 (1.4) difference, with the null hypothesis being correct, or because the number of subjects was too small to show Remote site: 8/15 (53%) Not any difference. numbers don't add up for Adjacent site: 6/15 (40%) reported "remote" sites: should be n=30. Remote Remote site, site, CAL CAL gain gain (1.3) 1.3 (1.3) 15 Not reported Not reported Not reported Not reported Not reported SD N/A Not reported Not reported 15 Not reported Not reported Not reported Not reported Not reported N/A 1.3 SD Not reported Not reported 10 patients 7.7 1.26 Not stated 5.78 1.38 SD Not reported Not reported N/A 1.02 0.26 SE 1.02 0.26 12 (10 included in this mean) 11.11 not given 12 0.54 0.18 SE 0.54 0.18 N/A 11 0.94 0.3 SE 0.94 0.3 12 (10 included in this mean) 11.11 not given 9 0.6 0.28 SE 0.6 0.28 N/A not given 11 1.1 0.29 SE 1.1 0.29 12 (10 included in this mean) 11.11 not given 10 0.45 0.2 SE 0.45 0.2 N/A 1.07 121 Not reported Not reported SD 1.01 0.82 124 5.63 1.06 123 Not reported Not reported SD 0.98 0.88 Not reported Reviewer analysis with some modifications from the submitted data by manufacturer 126 Not reported Not reported 1.01 Not reported Reviewer analysis with some modifications from the submitted data by manufacturer 11/15 (73%) Change in CAL (baseline given), mm ALL TREATED SITES Mean gain Mean SD CONTROL (or SE) (baselinegain, final) CONTROL 1.6 Study 103A: 5.56 SDs: 1.07 mm; (A); 1.60 Study (B) 103B: 5.21 128 237 Not reported Not reported SD 1.09 1.01 mm (A); 1.09 mm (B) 1.03 SDs: 0.82 (A); 1.03 (B) 126 250 5.23 5.63 mm: Study 103A; 5.23 mm: Study 103B 1.71 1.06 mm (A); 1.71 mmm (B) SD 0.99 0.98 (A); 0.99 (B) 32 Primary, seconday and subgroup analyses (12.9%) predetermined. Used patient as the unit of 0.87 mm (A); in test; observation. Primary outcome was change in 1.01 mm (B) 23 (9.2%) PD at sites initially >=5 mm in depth. No in control there analyses based on CAL. Many other group analyses based on PD reduction. Page 158 Locally-delivered antimicrobials: minocycline microspheres + SRP - Review authors’ judgment of each risk of bias item as low, unclear, or high for each included study Domain: Selection bias Random sequence generation Citation: Author, Year Support for judgment Henderson 2002 No details given Skaleric 2004 "randomized by envelope" Van Dyke 2002 No details given Performance bias Allocation concealment Masking of participants Masking of personnel Detection bias Were the groups treated the same except for the intervention? Review author's judgment Support for judgment Review author's judgment Support for judgment Review author's judgment Support for judgment Review author's judgment Support for judgment Review author's judgment Unclear No details given Unclear No High No High Yes Unclear No details given Unclear Details not provided Unclear No Unclear control subjects received irrigation High low No No High High Yes Yes Support for judgment Review author's judgment Low Yes Low Low No details given unclear Low Evaluator masked Low Unclear Unclear NDA 50-781: 103A No details given Unclear No details given Unclear Single masked but unknown who was masked Unclear Single masked but unknown who was masked Unclear No details given Unclear Single masked but unknown who was masked NDA 50-781: 103B No details given Unclear No details given Unclear Single masked but unknown who was masked Unclear Single masked but unknown who was masked Unclear No details given Unclear Single masked but unknown who was masked High Subjects in 2 of the 3 arms were: they had the powder (active or vehicel) reapplied at 3 and 6 months. The SRP only arm, however, didn't have something placed in the pocket at 3 and 6 months; but came in at the same periodicity for measurements. Rate as low because this is due to the intervention and overlaps with masking Williams as proxy for NDA "predetermined randomization plan" July 2015 Unclear No details given Unclear Not described as masked; NDA indicates single masking of outcomes assessors; however had third arm with placebo gel Unclear No Low Attrition bias Incomplete outcome data (Include percent Masking of outcomes lost to follow-up) assessment ≤10% low; 11-20% unclear; >20% high Examiners were blinded and it was they who assessed the outcome. Low Reporting bias Selective reporting Support for judgment Review author's judgment Support for judgment Review author's judgment No loss Low No bias detected Low Low No bias detected Low Unclear No bias detected Low % lost ranged from 5-8.9% Low Although CAL results were not published, they also were not specified as the primary outcomes measure Unclear % lost ranged from 4.7-8.7% Low Although CAL results were not published, they also were not specified as the primary outcomes measure Unclear Low Although CAL results were not published, they also were not specified as the primary outcomes measure. In fact CAL stated as included "as a safety assessment"; reported that "all groups experienced a mean gain in clinical attachment at 9 months, but the minocycline microsphere group showed a greater gain" when in fact, the difference was not statistically signficant Unclear Assumed no losses although not stated Up to 20% lost 8.4% of SRP and 4.8% of test group incomplete Page 159 Nonsurgical use of lasers: PDT diode laser + SRP - Study characteristics Citation: Author, Year Country Special population? (e.g. smokers) and other Severity of disease (e.g. data regarding inclusion refractory, mild, moderate) exclusion criteria for patients and teeth Test subject Control subject age (mean, age (mean, median, range median, range as described) as described) Control Control Dose/ duration/ frequency/ timing if applicable Test (typically adjunct) Test (typically adjunct) Dose/ duration/ frequency/ timing including simultaneous/ before/ after one appointment with hand instruments (Gracey curettes) SRP+laser wavelength of 670 nm For the photodynamic therapy 0.005% methylene blue was used as photosensitizer and activated with a laser / maximum power of 150 mW for 60 seconds Was standard Trial design counseling (split mouth / Duration of mentioned as part of parallel group / study control treatment? cross over) Adverse events reported No; non smokers Berakdar 2012 Chondros 2009 J Lasers med Sci Christodoulides 2008 Dilsiz 2012 Germany four teeth with probing depth ≥ SRP with curettes; All patients 5 mm and presence of BOP; Chronic periodontitis having four mean age: 59.3 ± 11.7 years (range 38received a professional no systemic disease; not teeth with at least one site ≥5 mm 74 years) tooth cleaning three weeks prior pregnant; no systemic probing depth and bleeding. to the treatment antibiotics within the last 6 months (a) chronic periodontitis, (b) no active periodontal treatment during the past 6 months, (c) presence of at least one site per quadrant exhibiting pocket depth of ≥4 mm with bleeding on probing, (d) good general Chronic periodontitis in supportive health without any signs of therapy with at least one site per Netherlands systemic disease, (e) no use quadrant with pocket depth ≥ 4 mm of antibiotics for the past with bleeding. 12 months and (f) no pregnancy; 3/9 smokers in control and 4/8 in test; Only sites with deep pockets (PPD≥4 mm) were treated subgingivally Patients referred to periodontal clinic, no treatment in past 2 years, no systemic diseases, Netherlands no pregancy, no antibiotics for past 12 months. No exclusion for smokers. Turkey Chronic periodontitis No systemic diseases, not Chronic periodontitis with at least 5 pregnant, not taking any teeth in every quadrant, presence medications, non-smokers, no of ≥4 non-adjacent teeth with PPD perio treatment in past 6 of ≥ 5 mm with bleeding and months, no caries or radiographic bone loss. restorations on selected teeth 48.3 (±7.9) 43.7 ± 7.3 years 50.6 (±9.2) 47.3 ±8.8 years 40.7 ± 7.3 years 40.7 ± 7.3 years 44.8 ± 5.6 yrs 44.8 ± 5.6 yrs HIV patients who had experienced highly active antiretroviral therapy; 33.3% were smokers Filho 2012 Brazil at least 4 sites presenting PPD >4 mm with BOP; not pregnant; no other systemic disease; no antimicrobials w/in the last 6 months Slight to moderate chronic periodontitis in HIV patients Smokers up to 10 cig/day Gianelli 2012 Italy at least 2 teeth w/ at least 1 site PD 4-10 w/ BOP in max quad (3-8 or 9-14); min of 5 teeth/quad Mod to severe chronic periodontitis 46.7 (range 25-65 yrs) Split mouth 6 months No undesirable effects were observed, and both therapies were tolerated well by the patients SRP/ mechanical debridement with a sonic scaler and supragingival cleaning and polishing YES. Subsequently, supragingival cleaning sonic scaler (Sonicflex®, was performed KaVo Dental GmbH, Biberach, Additionally a dye/laser system was applied. The with a rubber cup and a Germany) with power set to systemconsisted of a hand-held battery-operated diode laser SRP+Photodynam low-abrasive polishing 6000 Hz and water as coolant. The (HELBO® minilaser 2075 F dent, HELBO Photodynamic ic therapy at paste for both groups and instrumentation was Systems GmbH & Co KG, Grieskirchen, Austria). The laser selected site (≥4 repeated at 3- and 6terminated when the operator judged wavelength was 670 nm, and the power density 75 mW/cm2. mm PPD) month intervals. In the debridement to be the diode laser unit was used with an 8.5 cm-long flexible tip addition, self-performed adequately performed. Performed curved at an angle of 60°. Working time was 60 s per tooth plaque control measures under local anesthesia in 1 session were reinforced when indicated Parallel group 6 months there were no sites showing clinical signs of deterioration. Healing was uneventful in all patients. Neither pain nor any other discomfort was reported by any of the patients following both treatments SRP OHI individualized for every subject given at the Photosensitizer liquid applied with blunt needle starting from first appt; at 3 months SRP using hand instruments and SRP+Photodynam apical end of pocket and moving coronally; 3 minutes later all after clinical sonic instrumentation until operator ic therapy with pockets thoroughly rinsed with sterile saline to remove measurements, one believed root surfaces were diode laser excess photosensitizer; immediately after, diode laser with session of prophy adequately debrided and planed following SRP 670 nm wavelength and 75 mW power output equipped with including reinforcement of under local anesthesia in 1 session within 24 hours probe tip placed at depth of pocket and moved around tooth OH, supragingival for 1 minute debridement and tooth polishing Parallel group 6 months Healing was uneventful in all cases. No adverse effects, such as burning sensation or pain, related to the laser irradiation have been reported by any of the subjects 2 sessions with intervals of 7 days between sessions; tip of laser placed in perio pockets but laser beam not activated; full mouth supra and subgingival SRP with an ultrasonic instrument with A tip and medium intensity until clinician considered Note: although it states BEFORE SRP, does not seem likely tooth surfaces appropriately debrided SRP with recall professional / preliminary oral rinse for 30 s / pockets filled with 1% and planed; following SRP, all SRP+PDT GaAlAs prophy at 1 and 3 months after methylene blue solution / after 3 min photosensitizer rinsed supragingival teeth surfaces polished (diode) laser tx / no subgingival tx at recall out with saline / 100mW, 60s, 6J, 808 nm, 300 micron with rubber cup and point in fiberoptic tip combination with a dental paste; at 2nd session, SRP carried out using curettes and continued until researcher felt root surfaces were hard and smooth; then rinsed with sterilized phsiological saline (NaCl 9 w/v) solution SRP using ultrasonic device with recall weekly for first month and monthly until the 6th month; during recall supragingival prophy performed and OHI reinforced SRP with an ultrasonic SRP with ultrasonic scaler followed by the PDD with diode laser using methylene blue Smokers excluded 43.12 ± 8.2 years Brazil [2 treatments: TBO AND TBO+laser; exclude SRP+TBO arm] July 2015 3 nonadjacent sites with BOP and PD of 5-9 mm and at least 20 teeth excluding 3rd molars, age 35-55 Moderate to severe chronic periodontitis wavelength of 660 nm, 0.03 W power, methylene blue 0.01% lasting 133 seconds; spot size 0.07 cm2; fluence rate of 0.428 W/cm2, energy fluence of 57.14 J/cm2; pockets rinsed with saline after tx OHI at baseline; Split mouth OHI pre-tx and supragingival prophy monthly post-tx Split mouth photoablation: (1 W output power, continuous wave, 66.7 J/cm2), equipped with a 0.6 mm optical fibre touching the gingiva and removing junctionaln, sulcular and outer gingival Diode 810 nm epithelium.Tip moved at constant speed of 2.5 mm/s; with continuous wave, 1 threshold set at 80°C on the target (Bornstein 2004), to avoid W, 0.6 mm fiber SRP + sham laser (including undesired for photoablative All subjects received OHI methylene blue wash) +fullSham laser then SRP w/ Gracey heat-induced tissue damage. Split mouth, max 46.7 (range 25-65 and full-mouth prophy w/ mouth supragingival prophylaxis curettes; methylene blue rinsing then arch only, teeth 3yrs) Diode 635 nm ultrasound and/or hand by ultrasound and/or hand laser movements with laser off PDT: once weekly / (100 mW output power, continuous 8 and 9-14 continuous wave instruments instrumentation. wave), equipped with a 0.6 mm optic fibre / rinsed with the 100 mW, 0.6 mm photosensitizer agent methylene blue (0.3% w/v in water). / fiber for 2.5 mm/s speed (average time per tooth: 1 min. inside and 1 photodynamic min. outside the pocket / treatment was continued until normalization of the cytodiagnostic parameters, especially PMN (range: 4 –10 applications Theodoro 2011 Data for SRP+TBO+laser vs SRP Yes, patient compliance was required and monitored by routine plaque and gingival measurements 43.12 ± 8.2 years Inclusion range 35- Inclusion range 3555 55 SRP + toluidine blue O phenothiazine dye (TBO) TBO is 100 micrograms/mL / irrigation with insulin needle SRP+laser: 660 nm+ TBO All the patients participated in a program of oral hygiene instruction, professional power 30 mW, spot size 0.07 cm2, energy 4.5 J / After 1 min prophylaxis and of TBO irrigation, the laser optical fiber tip was positioned motivation weekly parallel to and in contact with the selected site. The laser was until day 30 after baseline delivered for 150 s at a power intensity of 0.4 W/cm2 and (at the beginning of the energy density of 64.28 J/cm2. treatments), then bimonthly until day 90, and monthly until day 180. Split mouth No adverse effects of laser therapy were observed 6 months or reported by the patients; none of the pts revealed after any major periodontal inflammatory symptoms after conclusion of instrumentation during the entire study; no tx complications such as infections, suppuration, or abscesses were observed 6 months No complications reported 1 year Stated that no adverse events occurred 6 months No postoperative complications, abscesses or infections were observed during the whole study period. Page 160 Nonsurgical use of lasers: PDT diode laser + SRP - Updated literature Citation: Author, Year Alweili, 2013 Betsy, 2014 Country Special population? (e.g. smokers) and other data regarding inclusion exclusion criteria for patients and teeth Jordan The exclusion criteria were systemic diseases that could influence the outcome of periodontal therapy, including antiphlogistics, bleeding stimulating pharmaceuticals, or intake of systemic antibiotics within the last 6 months. The inclusion criteria were as follows: previously untreated chronic periodontitis, at least one premolar and one molar in every quadrant with a minimum of four teeth each, and at least one tooth with attachment loss of ≥4 mm in every quadrant. India The inclusion criteria followed for patient selection comprised of (a) probing pocket depths (PPD) between 4 and 6 mm at least in two different quadrants of the mouth, (b) a minimum of 20 teeth, (c) age between 18 and 65 years (both males and females), (d) single rooted teeth, good general health without any signs of systemic disease, (e) no use of antibiotics for the past 6 months, (f) female patients were not pregnant or lactating, (g) non-smoking and (h) nonallergic to methylene/toludine blue. Severity of disease (e.g. refractory, mild, moderate) at least one tooth with attachment loss of ≥4 mm Not stated Test subject age (mean, median, range as described) 39.9 (13.5) 40.8 (8.3) Control subject age (mean, median, range as described) 39.4 (13.7) 38.4 (9.6) Control SRP SRP Control Dose/ duration/ frequency/ timing if applicable SRP of all periodontally involved teeth using both hand instruments (Gracey Curettes; Hu-Friedy, Leimen, Germany) and a piezoelectric ultrasonic handpiece (Sirosonic L; Sirona, Bensheim, Germany) with a slim-line styled scaler tip (Perio Pro Line; Sirona) by the same clinician. hand scalers and universal curettes (Hu-Friedy) and ultrasonic scaler (Woodpecker ). Full-mouth supragingival and subgingival scaling was performed at all sites within 24 h including the evaluated sites until the operator felt that tooth and root surfaces were adequately debrided and planed. Test (typically adjunct) SRP+PDT Test (typically adjunct) Dose/ duration/ frequency/ timing including simultaneous/ before/ after Initially, the subjects received full mouth SRP diode laser (wavelength, 660 nm; output and oral hygiene power, 100 mW; Helbo Photodynamic instructions. Briefly, Systems, Grieskirchen, subjects were trained in Austria) in combination with a dedicated the Bass technique. A soft photosensitizer dye (phenothiazine bristle toothbrush and a chloride; Helbo Photodynamic Systems). fluoride The photosensitizer dye allowed reacting toothpaste were provided for at least 1 to 3 min according to the along to them. The depth of the pocket. Laser application patients were was performed circumferentially at six called for to check their sites per tooth, 1 min per tooth in six adherence to the clinical areas each one at 10 s.. instructions, as well as to supragingival control every 2 weeks. The photosensitizer used consisted of freshly prepared 3,7-bis (dimethyl- amino) phenazathionium chloride trihydrate [methylene blue (MB) M9140; Sigma-Aldrich, St. Louis, MO, USA] suspended in double distilled water at a concentration of 10 mg/ml. SRP+PDT Was standard Trial design counseling (split mouth / Duration of mentioned as part of parallel group study control treatment? / cross over) diode laser operating at 655 nm with a CW output power of 1 W (CSP). Two millilitre of MB was applied topically to sites with 4–6 mm pocket depth for 60 s using a syringe / The aPDT treatment was performed at a continuous laser power density of 60 mW/cm2, with top-hat energy distribution for 60 s, at each mesiobuccal, distobuccal, mesiolingual or distolingual site with 4–6 mm pockets on selected teeth all subjects were initially enrolled in an oral hygiene program and were given oral hygiene instructions that included twice daily brushing before they returned for treatment after 1 week. Adverse events reported split-mouth; 2 quadrants control and 2 quadrants test 1 year No adverse effects of aPDT were observed or reported by the patients.None of the patients revealed any major periodontal inflammatory symptoms after instrumentation during the entire study. Postoperative complications, such as infections, suppuration, or abscesses, were not observed. Parallel 6 months Healing was uneventful in all cases and no adverse effects, such as discomfort, burning sensation, or pain related to the laser irradiation, were reported by any of the subjects. Parallel 6 months The subjects did not report adverse effects after therapies. NON SMOKERS Luchesi, 2013 Brazil July 2015 Exclusion criteria were pregnancy, lactation, current smoking and smoking within the past 10 years, antibiotic therapies in the previous 6 months, use of long-term treatment of class II furcation sites administration of antiin chronic periodontitis subjects. / inflammatory and and chronic perio one buccal or immunosuppressive lingual class II furcation with medications, use of mouth probing pocket depth (PPD) ≥ 5 rinses containing mm and bleeding on probing (BoP). antimicrobials in the preceding 2 months, systemic conditions reported during anamneses that could affect the progression of periodontitis (e.g. diabetes mellitus) and SRP in the preceding 6 months. 50.75 ( 8.18) 50.24 (10.89) SRP + non-activated laser/only photosensitizer (control group Scaling and root planing was performed using periodontal curettes (Gracey, Hu-Friedy, Chicago, IL, USA) and an ultrasonic device SRP +activated laser with photosensitizer The laser system included a handheld battery-operated diode laser (Thera Lase – DMC, S~ao Paulo, Brazil) with a wavelength of 660 nm, a power output of 60 mW and energy dose of 129 J/cm2, together with methylene blue as a photosensitizer (10 mg/ml) patients were submitted to dental calculus removal, exodontia, provisional restorations, and SRP in all non-experimental sites At the end of each appointment, supragingival prophylaxis was performed. Page 161 Nonsurgical use of lasers: PDT diode laser + SRP - Outcomes data Citation: Author, Year Berakdar 2012 Outcome measure CAL, mm Time period Other time No. Sites treated for data periods for per mouth / No. presented in which data sites averaged this are per tooth abstraction available 6 months Data recorded at 6 sites per tooth with at least one tooth used baseline, 1, 3 as test or control. No months mention of total number of teeth involved in study. Test sample size Baseline Test mean Baseline 22 people 8.1 Test Test SD or Mean gain Control Test SD or SE sample Test mean at SE (list Mean SD TEST sample (list value) size at end end of test value) at SD or SE? (or SE) (Baselinesize Baseline of test period end of test gain, TEST final) Baseline period period 1.3 22 people not recorded, only plot graph shown. No actual numbers given for results. NA NA Range read Plot graph off figure: 1.5indicates 2.5 4.0. No SD mm gain in or SE CAL available 22 people Control mean Baseline 7.2 Control Control Control SD or SE sample mean at end (list value) size at end of test Baseline on test period period 1.2 22 people not recorded, only plotted Control SD Mean gain or SE (list Mean SD (or CONTROL SE) gain, value) at SD or SE? (baselineCONTROL end of test final) period NA NA Caries data range read off Plot graph figure: 0.5indicates 2.0 3.5. No SD Not reported mm gain or SE available Christodoulides 2008 CAL 6 months 6 months 10 sites per mouth for baseline and each group, no 12 patients with 3 months mention of #sites per 10.7 ± 3.2 sites tooth. 6.8 56 test sites (12 patients) and 57 baseline and control sites (12 3 months patients). No mention of # of sites per mouth. 4.1 56 sites in 12 patients The nonparametric Wilcoxon test for paired samples was used for comparison of the effect of the two treatments (p ≤ 0.05). Power calculation; Student’s t-test was employed for continuous variables (clinical measurements) after the normality of the data distribution had been confirmed. Likewise, the significance of the difference within each group before and after treatment was evaluated with the pairedsamples t-test CAL - distance in millimetres from a fixed reference Chondros 2009 J point (cemento-enamel Lasers med Sci junction or the border of a restoration) to the bottom of the probeable pocket, at the experimental sites) Statstical analysis notes 0.9 0.5 12 56 sites in 12 patients 6.1 3.4 0.9 0.6 SD SD 0.7 0.7 0.7 0.3 12 57 sites in 12 patients 7.1 4.5 0.9 1 12 57 sites in 12 patients 6.6 4 0.9 1 SD SD 0.5 0.5 0.6 0.5 Not reported The calculation of the sample size determined that ten subjects per treatment group would provide 80% power to detect a true difference of 1.0 mm between test and control, using probing depth (PD) reduction in pockets as the primary outcome variable, assuming that the common standard deviation would be 0.8 mm. Power calculation; Student’s t-test was employed for continuous variables (clinical measurements) after the normality of the data distribution had been confirmed. Likewise, the significance of the Not reported difference within each group before and after treatment was evaluated with the paired samples ttest Power calculation to detect true difference of 1 mm with SD of 0.8 for PD Dilsiz 2012 Filho 2012 Gianelli 2012 CAL (mm) CAL 6 months 6 months CAL, 6 sites per tooth; minimum 5 teeth per quadrant 1 year CAL, mm 6 months baseline 45 days, 3 and 6 months 3 sites per person; 144 teeth; 1 site per person per regimen (3 regimens) 24 people All teeth with at least 12 pts, all teeth 1 site with PD ≥ 4 with ≥ 4 mm PD, mm; 6 sites/tooth 6 sites/tooth 0,15,30,45,60 5-6 test teeth and 5-6 ,90 and 365 control teeth per for patient; 6 sites per cytodiagnosti tooth and values were c data averaged 7.67 4 0.56 1.1 24 people 6.13 12 pts, all teeth with ≥ 4 mm PD, 6 sites/tooth 2.6 0.99 1 SD SD 1.54 1.1 Not reported Not reported 24 people 7.5 12 pts, all teeth with ≥ 4 mm PD, 6 sites/tooth 3.7 0.72 0.9 24 people 6.04 12 pts, all teeth with ≥ 4 mm PD, 6 sites/tooth 3.6 1 0.8 SD SD 1.5 0.88 Not reported Not reported Not reported Not reported Kruskal-Wallis test chosen for multiple comparisons of the groups in mean CAL / Wilcoxian signed rank test for differences in mean clinical parameters for baseline and 6 months; Mann Whitney U used to compare the groups The ANOVA/Tukey was used for statistical analysis; power calculation; per protocol analysis; patients statistical units; student's t test for clinical parameters This analysis indicated that with 12 subjects the study would have an 80% power to detect a 1 mm difference in the CAL in the two groups 26 people; 150 teeth 5.6 0.2 26 people; 150 teeth 3.1 0.2 SE Not reported Not reported 26 people; 150 teeth 5.6 0.2 26 people; 150 teeth 4.8 0.2 SE Not reported Not reported Not reported The subject’s quadrant was assumed as a test unit for statistical comparison.The clinical parameters were compared by Student’s t-test for paired values 33 6.52 2.11 33 4.96 2.07 SD Not reported Not reported 33 6.23 1.25 33 4.25 1.73 SD Not reported Not reported Examiner calibration procedure / power and sample size calculated at 81% / data tested for normality Theodoro 2011 Data for SRP+TBO+laser vs SRP [2 treatments: TBO AND TBO+laser; exclude SRP+TBO arm] July 2015 2, 3 and 6 months 3 sites/mouth, 1 site/tooth N/A Page 162 Nonsurgical use of lasers: PDT diode laser + SRP - Updated literature Citation: Author, Year Outcome measure Alweili, 2013 CAL, mm Time period for No. Sites treated data presented in per mouth / No. this abstraction (as sites averaged per close to 9 months tooth as possible) 1 year Not described; at least one tooth with attachment loss of ≥4 mm per quadrant Test sample size Baseline Test mean Baseline 21 9.22 Test Test SD or Mean Mean SD Test SD or SE sample Test mean at SE (list difference (or SE) (list value) size at end end of test value) at SD or SE? TEST (final- difference, Baseline of test period end of test baseline) TEST period period 2.16 16 people, 73 sites 7.74 2.02 SD 1.48 1.89 Control sample size Baseline Control mean Baseline Control SD or SE (list value) Baseline 21 9 2 Control Control SD Control sample or SE (list mean at end size at end value) at SD or SE? of test on test end of test period period period 16 people, 63 sites 8.87 2.13 SD Mean difference CONTROL (finalbaseline) 0.13 Mean SD (or SE) Caries data difference, CONTROL 1.7 No caries data Statstical analysis notes Used Mann-Whitney for statistical significance between treatments Power calculation performed, minimum effect size (before-after) set at 0.5 mm with SD = 0.6mm Subject-level analyses Betsy, 2014 median CAL, mm 6 months PDT applied to sites with PD 4-6 mm 44 6.5 Min-max, IQR: 5.0-8.0; 1.7 44 (ITT) 4 Min-max, IQR: (2.6–7.0; 2.0) IQR Not reported Not reported 44 6 Min-max, IQR: (4.2–8.0; 1.7) 44 (ITT) 4.5 Min-max, IQR: (2.0–7.0; 2.0) IQR Not reported Not reported Not reported Intention to treat analysis; LOCF method the data distribution of clinical parameters in this study did not obey the Gaussian law by KolmogorovSmirnov test (p < 0.05), nonparametric methods were used for analysing the data The minimum clinically significant value (d) considered was 1 mm Luchesi, 2013 relative CAL (RCAL) – the distance from the stent to the bottom of the periodontal pocket 6 months Not stated 21 people 10.56 1.79 16 people 9.78 2.33 SD 0.78 1.54 21 people 10.43 2.66 21 people 9.5 2.14 SD 1 1.69 Not reported Once normal distribution were obtained, repeated measures analysis of variance (ANOVA) and Tukey’s test were used to detect intra-group and inter-group differences in clinical parameters (PGM, PPD and RCAL) Appears that data analyzed per tooth/site and not per person July 2015 Page 163 Nonsurgical use of lasers: PDT diode laser + SRP - Review authors’ judgment of each risk of bias item as low, unclear, or high for each included study Domain: Selection bias Random sequence Citation: Author, Year Support for judgment Berakdar 2012 randomization list Chondros 2009 J Coin toss Lasers med Sci Christodoulides Coin toss 2008 Dilsiz 2012 computer generated table Teeth randomlly Filho (Noro Filho) allocated using a 2012 computer generated list Performance bias Allocation concealment Review Support for author's judgment judgment Low No details given Review author's judgment Unclear Masking of participants Masking of personnel Were the groups Attrition bias Reporting bias Incomplete outcome data Selective reporting No - single blinded Review author's judgment High No-single blinded Review author's judgment High Low No details given Unclear Not described Unclear Not described Unclear Yes Low No details given Unclear Not described Unclear examiner blind. Clinician not. High Yes Low No High Yes Low "double blind" Low No loss; outcomes complete Low No bias detected Low Support for judgment Support for judgment Support for judgment Yes Detection bias Masking of outcomes Review Support for author's judgment judgment examiner Low not involved Examiner Low masked Examiner Low masked Review author's judgment Low Low Low Support for judgment No losses No losses to follow up No loss; outcomes complete Review author's judgment Low Support for judgment Review author's judgment No bias detected Low Low No bias detected Low Low No bias detected Low Low No details given Unclear Laser placed in pocket but not activated Low Code not broken until end of study Low "Double blinded" but method not described Unclear "Double blinded" but method not described Low Yes Low "Double blinded" but method not described Low All patients finished and were reported Low All data reported Low Gianelli 2012 randomization claimed, but method not described Unclear Sequentially numbered opaque sealed envelopes Low Sham laser Low Not masked High Yes Low Blinding described; unaware of tx Low No losses to follow up Low All data reported Low Theodoro 2012 Computer generated table, tx sequence also randomized Low No details given Unclear No detail provided Unclear Examiner blinded Low Yes Low Examiner blinded as to treatment Low 4 patients exited prior to data collection Low All data reported Low July 2015 Page 164 Nonsurgical use of lasers: PDT diode laser + SRP - Updated literature Selection bias Domain: Random sequence generation Citation: Author, Year Support for judgment Alweili, 2013 quadrants were assigned to different groups according to a computergenerated random number table Betsy, 2014 Tippet’s 2-digit random number table Luchesi, 2013 July 2015 by a computergenerated list, Review author's judgment Performance bias Allocation concealment Masking of participants Masking of personnel Support for judgment Low The sequence was concealed until the interventions were assigned Low opaque sealed envelopes, which were sequentially numbered. Low The randomization code was not broken until all data had been collected Review author's judgment Low Low Low Support for judgment Not described Not described Sham laser Review author's judgment Unclear Unclear Low Support for judgment Not described Not described Not possible / described Were the groups treated the same except for the intervention? Detection bias Attrition bias Reporting bias Masking of outcomes assessment Incomplete outcome data (Include percent lost to follow-up) ≤10% low; 11-20% unclear; >20% high Selective reporting Review author's judgment Support for judgment Review author's judgment Support for judgment Review author's judgment Support for judgment Review author's judgment Support for judgment Review author's judgment Unclear Reinforcement regarding oral hygiene was provided on an individual basis by dental hygienist not involved in the clinical examination Unclear blinded examiner Low 6/21 lost (24%) High None noted Low Low All clinical data collected by periodontist blinded to study procedure Low 8/88 lost; ITT analysis Low None noted Low High clinical attachment level (CAL) change defined as the primary outcome variable Low Unclear Unclear No differences noted No differences noted Low Examiner blinded to therapies Low 24% for treatment group Page 165 Nonsurgical use of lasers: non-PDT laser + SRP - Study characteristics Citation: Author, Year Country Special population? (e.g. smokers) and other Severity of disease (e.g. data regarding inclusion refractory, mild, moderate) exclusion criteria for patients and teeth Alves 2012 Caruso 2008 Brazil Italy Systemically healthy subjects only, no medications or antibiotic use in past month, no perio therapy in past 3 months, at least 10 teeth per arch. No mention of smoking status. Control Control Dose/ duration/ frequency/ timing if applicable SRP supra- and subgingival ultrasonic scaling of all teeth except for the experimental teeth; One day after SRP and polish, laser 46.8±8.11 years (range 37–64 years) experimental/control teeth procedure used on the control tooth received subgingival SRP under without activation of the laser in a anesthesia followed by tooth sham procedure. Laser sham was polishing repeated one week after SRP No; non-smokers only single-rooted teeth with pocket depth >5 mm; no perio tx or antibiotics for the previous 6 months; no systemic pathogies; no pregnant women Test subject Control subject age (mean, age (mean, median, range median, range as described) as described) Severe chronic periodontitis Chronic periodontitis having at least two sites in contralateral sextants with probing depths ≥ 5 mm interproximally of the same arch and within 2 mm difference. Not stated Not stated Hand scaling and RP with a curette by same person. Test (typically adjunct) Dose/ duration/ frequency/ timing including simultaneous/ before/ after Test (typically adjunct) "high intensity" 808±5 nm diode laser Was standard Trial design counseling (split mouth / Duration of mentioned as part of parallel group / study control treatment? cross over) 20 s, in two isolated appointments, 1 day after SRP and again 1 week later. The laser was used in the continuous all subjects received oral mode, with 1.5 W and power density of 1,193.7 W/cm2. 400hygiene instruction (OHI) μm diameter fiber optic device. The mean energy loss in this study was around 20%. Power meter used. Adverse events reported Split mouth contralateral teeth, 1 test & 1 control 6 months Not reported Split mouth 6 months Not reported mechanical debridement by the same expert periodontist, using (Valure S9- Lasering Medical LaserGracey curettes until the operator Modena; Italy) 980 nm at a power output of 2.5 W in pulsed All patients were achieved a hard, smooth and mode (30 Hz, pulse duration 10 ms) after SRP / The optic instructed with oral calculus-free root surface. One SRP+Diode laser; fiber of 400 μm was moved from the coronal to the apical side hygiene treatment appointment for all of the pocket in parallel paths with an inclination of instructions after consent subjects with follow-up for plaque approximately 20°. Each pocket of the test group was lased to participate control at 4,8,12 weeks and final eval for 30 s twice, with a 60 s interval at 6 months Nonsurgical use of lasers: non-PDT laser + SRP - Updated literature Citation: Author, Year Saglam, 2014 Ustun, 2014 July 2015 Country Special population? (e.g. smokers) and other data regarding inclusion exclusion criteria for patients and teeth Turkey Exclusion criteria were periodontal treatment received for the last 1 year; systemic diseases that could influence the outcome of the therapy, pregnancy, smoking, immunosuppressive chemotherapy; and use of antibiotics and antiinflammatory drugs for the last 6 months. Patients were included if they had at least 14 teeth with at least two teeth with ≥5 mm probing depth at each quadrant. Turkey Severity of disease (e.g. refractory, mild, moderate) at least two teeth with ≥5 mm probing depth at each quadrant Inclusion criteria were the presence of at least two incisors or canines at two quadrants (mandible or maxilla) with periodontal Not stated, but the selection criteria pocket depths between 4 and 7 mm. (4-7 mm pockets) and the Exclusion criteria were a history of any systemic diseases that attachment level at the start (about could affect the periodontal therapy outcome (e.g., diabetes 4.7 mm) would indicate moderate mellitus, cancer, metabolic or endocrine diseases), smoking, chronic periodontitis as the most dental treatment in the past 6 months, antibiotic medication during likely diagnosis the 6 months preceding the study, and related teeth with restoration. Test subject age (mean, median, range as described) 42.13±9.05 Control subject age (mean, median, range as described) 40.83±7.64 Control SRP Control Dose/ duration/ frequency/ timing if applicable Full-mouth subgingival scaling and root planing under local anesthesia was performed in a single appointment for each patient in all groups using an ultrasonic scaler and hand instruments. Test (typically adjunct) SRP followed by diode laser In order to control for the same conditions, pockets were also rinsed with saline after SRP in the control group. SRP one week later, SRP treatment alone. The entire quadrant was treated according to the procedure, but only one incisor or canine was sampled. Was standard Trial design counseling (split mouth / Duration of mentioned as part of parallel group study control treatment? / cross over) Laser treatment was performed by using a 940 nm indium–gallium– All patients received oral aluminum–phosphate diode laser (Ezlase, hygiene instructions and Biolase, USA). The periodontal pocket supragingival was set at 1.5 W with a pulse interval of scaling in a single 20 ms and pulse length of 20 ms appointment 1 week apart delivering 20 s/ cm2 and 15 J/cm2 of before treatment. energy. Irradiation was accomplished with a 300 μm fiber optic delivery system. Parallel 6 months Adverse events reported No adverse effects, such as discomfort, burning sensation, dentin hypersensitivity, or pain related to the laser irradiation were reported by any of the subjects. Both patients and the operator wore protective glasses during laser application. One week after subgingival scaling; SRP and laser treatment; subgingival scaling with a combined use of hand (Hu-Friedy, Chicago, IL) and ultrasonic instruments 40.23 +/– 10.18. Test (typically adjunct) Dose/ duration/ frequency/ timing including simultaneous/ before/ after SRP+diode laser All patients received initial periodontal therapy 810 nm diode laser (Fotona XD-2, Fotona consisting of thorough d.d., Slovenia). Laser parameters were oral hygiene instructions Ppeak = 2.5 W, duty cycle ½, Pavg = and full-mouth 1.25W, 20Hz and 80 sec/tooth (mesialy, supragingival distally, lingually and buccally; 20 scaling sec/site). Laser was applied before root planing Healing was uneventful in all cases. No adverse effects related to the laser irradiation were reported. split mouth 6 months During the laser treatment, protective eyeglasses were used by patient and operator. Page 166 Nonsurgical use of lasers: non-PDT laser + SRP - Outcomes data Citation: Author, Year Time Other time period for No. Sites treated periods for data per mouth / No. Outcome measure which data presented sites averaged are in this per tooth available abstraction 6 months 6 weeks One pair of contralateral single-rooted teeth was chosen from each subject; 6 sites per tooth measured and "deepest site of each experimental tooth as defined as the experimental site" 6 months CAL only recorded at baseline and 6 months Recorded data with customized stent at interproximal sites only. Had 38 teeth involved as 19 pairs. CAL Alves 2012 Caruso 2008 Not stat sig between groups; 1.0 mm difference CAL (referring to the enamel-cementum junction) / Williams probe Test Test SD Test sample Test sample Test mean at or SE (list size mean size at end end of test value) Baseline Baseline of test period Baseline period Test SD or Mean gain Control SE (list Mean SD TEST sample value) SD or SE? (or SE) (Baselinesize at end gain, TEST final) Baseline of test period 37 people 6.91 1.94 36 people 5.33 2.13 SD 13 patients with 19 sites 7.123 0.9 13 patients 5.089 0.8 SD 1.7 1.72 Not reported Not reported Control mean Baseline Control Control Control SD or Control SD or SE sample mean at end SE (list value) (list value) size at end of test at end of test Baseline on test period period period SD or SE? 37 people 6.5 1.74 36 people 4.61 1.88 SD 13 patients 6.912 1 13 patients 5.123 0.9 SD Mean gain Mean SD (or CONTROL SE) gain, (baselineCONTROL final) 2.1 1.64 Caries data Statstical analysis notes N/A power calculation to determine 1.0 difference in CAL; ANOVA to determine differences between averages of groups and time periods; Newman Keuls test for multiple comparisons Not reported Not reported Not reported Wilcoxon signed-rank test for paired samples. No statistical data given for P- values, only shown for probing depth not CAL. Nonsurgical use of lasers: non-PDT laser + SRP - Updated literature Citation: Author, Year Outcome measure Whole mouth CAL, mm Time period for No. Sites treated data presented in per mouth / No. this abstraction (as sites averaged per close to 9 months tooth as possible) 6 months Saglam, 2014 The maxillary anterior region (maxillary incisors, canine, and premolar teeth) was used as the test site for the evaluation of site-specific clinical parameters The entire quadrant was treated according to the procedure, but only one incisor or canine was sampled Site specific CAL, mm Ustun, 2014 July 2015 CAL, mm Clinical parameters were performed at six sites per tooth (mesiobuccal, mid-buccal, distobuccal, mesio-palatal, mid-palatal, and disto-palatal). 6 months CAL measurements were recorded at six points (mesio-buccal, mid-buccal, disto-buccal, mesiolingual, mid-lingual, and disto-lingual) around each tooth with a manual periodontal probe Test sample size Baseline Test mean Baseline Test Test SD or Mean Mean SD Test SD or SE sample Test mean at SE (list difference (or SE) (list value) size at end end of test value) at SD or SE? TEST (final- difference, Baseline of test period end of test baseline) TEST period period Control sample size Baseline Control mean Baseline Control SD or SE (list value) Baseline Control Control SD Control sample or SE (list mean at end size at end value) at SD or SE? of test on test end of test period period period Mean difference CONTROL (finalbaseline) Mean SD (or SE) Caries data difference, CONTROL Statstical analysis notes CAL difference in the change between methods of 1 mm 15 2.7 ±0.4 15 1.7 ±0.2 SD Not reported Not reported 15 2.8 ±0.6 15 1.9 ±0.4 SD Not reported Normality tests performed and statistical analyses adjusted based on the results Not reported No caries data 15 5.3 ±0.6 15 1.9 ±0.4 SD Not reported Not reported 15 5.2 ±1.1 15 2.5 ±0.9 SD Not reported Not reported 21 4.68 0.75 19 3.01 0.67 SD Not reported Not reported 21 4.75 0.78 19 3.46 0.71 SD Not reported Not reported No stat sig differences between groups Power based on 1 mm difference in PPD Not reported Stat sig difference at end between groups Page 167 Nonsurgical use of lasers: non-PDT laser + SRP - Review authors’ judgment of each risk of bias item as low, unclear, or high for each included study Domain: Selection bias Random sequence generation Performance bias Allocation concealment Masking of participants Were the groups treated the same except for the intervention? Masking of personnel Citation: Author, Year Support for judgment Review author's judgment Support for judgment Review author's judgment Support for judgment Review author's judgment Support for judgment Review author's judgment Alves 2012 Coin toss after initial tx Low 2nd examiner did coin toss Low "Double blinded" with sham Low Examiner was not aware of tx; 2nd examiner did coin toss Low Caruso 2008 Coin toss Low No details given Unclear Not described Unclear Not described Unclear Detection bias Attrition bias Reporting bias Masking of outcomes assessment Incomplete outcome data (Include percent lost to follow-up) ≤10% low; 11-20% unclear; >20% high Selective reporting Support Review Support for for author's judgment judgment judgment Initial tx same for all teeth; study Blinded Low SRP same examiner for both teeth Yes Low Not described Review author's judgment Support for judgment Review author's judgment Support for judgment Review author's judgment Low 1 subj excluded for missing appt Low All data reported Low Unclear not reported Unclear Stat sig not reported. No p values reported for CAL table Unclear Nonsurgical use of lasers: non-PDT laser + SRP - Updated literature Selection bias Domain: Random sequence generation Performance bias Allocation concealment Masking of participants Masking of personnel Review author's judgment Support for judgment Saglam, 2014 Methods not described Unclear Patients did not know which group they were assigned to until interventions were performed Unclear Not masked High Ustun, 2014 coin toss Low Not described Unclear Not masked High Citation: Author, Year July 2015 Support for judgment Review author's judgment Support for judgment Review author's judgment Were the groups treated the same except for the intervention? Review author's judgment Support for judgment Not masked Not masked Support for judgment Detection bias Attrition bias Reporting bias Masking of outcomes assessment Incomplete outcome data (Include percent lost to follow-up) ≤10% low; 11-20% unclear; >20% high Selective reporting Review author's judgment Support for judgment Review author's judgment Support for judgment High No differences noted High No differences noted Review author's judgment Support for judgment Review author's judgment Low Statistician masked Low All subjects completed the entire study Low CAL primary outcome and results reported Low Low Masked Low 2/21 lost; 10% Low None noted Low Page 168 Nonsurgical use of lasers: Nd:YAG laser + SRP - Study characteristics Citation: Author, Year Country Eltas 2012 Turkey Special population? (e.g. smokers) and other Severity of disease (e.g. data regarding inclusion refractory, mild, moderate) exclusion criteria for patients and teeth No periodontal therapy in past Moderate chronic periodontitis with 12 months, no systemic at least two qaudrants of ≥ 3 teeth diseases, no antiobiotics in with 4 - 6 mm pocketing and past 6 months, no pregnancy, radiographic bone loss. no restored teeth Test subject Control subject age (mean, age (mean, median, range median, range as described) as described) Control Control Dose/ duration/ frequency/ timing if applicable Test (typically adjunct) Test (typically adjunct) Dose/ duration/ frequency/ timing including simultaneous/ before/ after median age of 46.1±8.3 years SRP full mouth / supra and subgingival with combined use of hand and ultrasonic instruments in 1 session under local anesthesia SRP+Nd:YAG laser (NDL) Subsequent to SRP / 1.0 W NDLlllll (wavelength 1064 nm, 100 mJ, 10 Hz) / optic fiber 200 micron diameter / fiber tip inserted bottom of perio pocket and slowly moved from apical to coronal in sweeping motion / 120 s exposition time Was standard Trial design counseling (split mouth / Duration of mentioned as part of parallel group / study control treatment? cross over) OHI Split mouth Adverse events reported 9 months Not reported 6 months None reported; minimal post-op pain reported Split mouth No Neill 1997 U.S. probing depths > 4 mm with radiographic bone loss moderate to severe adult periodontitis (PD 4mm or greater) average age of 44 years (range 3353) SRP Mechanical SRP was used to remove calculus and other deposits from the root surfaces to achieve the SRP+low powered Sulcular debridement using energy/pulse of 80 mJ, repetition endoint of a smoot, glass-like root pulsed Nd:YAG rate of 25 Hz and average power of 2.0 W, average time of 2 surface. Ultrasonic instrumentation laser min. Depth of pocket influenced time of laser application. was included where appropriate, and the site was irrigated with a sterile saline solution. quadrants Yes; but only mentioned randomly for no treatment group assigned to one of (not SRP group or laser three experimental group); patient plaque groups: if 4th control instructions quadrant had an elegible site, it was assigned to the laser group Nonsurgical use of lasers: Nd:YAG laser + SRP - Updated literature Citation: Author, Year Eltas, 2012 Country Special population? (e.g. smokers) and other data regarding inclusion exclusion criteria for patients and teeth Severity of disease (e.g. refractory, mild, moderate) Turkey (1) Patients with generalized moderate CP with the presence of at least 2 teeth with PD between 4 and 6mm and radiographic signs of bone loss per quadrants were included.(2) Smokers were identified as smoking > 10 cigarettes per day for > 5 years, whereas nonsmokers were identified as never having smoked. Standard exclusions (tx in prior 12 months; systemic diseases; systemic or topical antibiotics or steroidal/nonsteroidal anti-inflammatories within last 6 months; currently pregnant or breastfeeding; teeth with tx that could affect perio outcomes moderate July 2015 Test subject age (mean, median, range as described) Control subject age (mean, median, range as described) mean age of 43.5 years old (range: 32–52 years) Control Control Dose/ duration/ frequency/ timing if applicable Test (typically adjunct) Test (typically adjunct) Dose/ duration/ frequency/ timing including simultaneous/ before/ after SRP+ placebo subgingival SRP with the combined use of hand and ultrasonic instruments under local anesthesia in the same session. SRP + Nd:YAG laser 1.0 W NDL (wavelength 1,064 nm, 100 mJ, 10 Hz), exposure time of 120 sec Was standard Trial design counseling (split mouth / Duration of mentioned as part of parallel group study control treatment? / cross over) initial periodontal therapy consisting of thorough oral hygiene instructions and full-mouth supragingival split mouth for effect of laser; parallel group for effect in smoking vs non smoking Adverse events reported Adverse outcomes were not assessed 6 months protective eyeglasses were worn by the patient, the operator, and the assistants Page 169 Nonsurgical use of lasers: Nd:YAG laser + SRP - Outcomes data Citation: Author, Year Eltas 2012 Neill 1997 Outcome measure Time period Other time No. Sites treated for data periods for per mouth / No. presented in which data sites averaged this are per tooth abstraction available CAL (cementoenamel junction and deepest aspect of pocket,) This study was performed on 40 teeth (probing depth between 4 and 6 mm) from 20 patients / 6 surfaces per tooth / one site per person per test CAL gain, mm 9 months baseline, 3 months 6 months 1 week, 1 month, 3 months 10 pts, 186 total teeth: 91 laser, 49 SRP and 46 NT 744 total sites: 364 laser, 196 SRP and 184 NT Test sample size Baseline Test mean Baseline Test Test SD or Mean gain Control Test SD or SE sample Test mean at SE (list Mean SD TEST sample (list value) size at end end of test value) at SD or SE? (or SE) (Baselinesize Baseline of test period end of test gain, TEST final) Baseline period period 20 patients 5.24 1.51 20 patients, 20 teeth 10 people; 91 teeth Not reported Not reported 10 people; 91 teeth 2.83 Not reported 1.44 SD Not reported Not reported Control Control Control SD or SE sample mean at end (list value) size at end of test Baseline on test period period Control mean Baseline 20 patients, 20 teeth 5.13 1.21 20 patients, 20 teeth 4.03 Control SD Mean gain or SE (list Mean SD (or CONTROL SE) gain, value) at SD or SE? (baselineCONTROL end of test final) period 1.27 SD NOTE: different from figure 1.9 10 people; 49 Not reported teeth Not reported 10 people; 49 Not reported teeth Not reported Not reported Statstical analysis notes Kruskal–Wallis and Mann–Whitney U tests were used to determine the significance of the Not reported Not reported Not reported differences between groups, while Friedman and Wilcoxon tests were used to determine the significance of the differences within groups 1.0 1.1 Not reported Not reported Caries data Note: different from figure 1.7 Not reported Comparisons by individual tooth utilized ANOVA, Kruskal-Wallis nonparametric ANOVA, and chisquare tests; SD or SE not defined; figure data different from text data Nonsurgical use of lasers: Nd:YAG laser + SRP - Updated literature Citation: Author, Year Outcome measure Time period for No. Sites treated data presented in per mouth / No. this abstraction (as sites averaged per close to 9 months tooth as possible) SMOKERS CAL, mm Eltas, 2012 6 months NONSMOKERS CAL, mm July 2015 208 teeth (with PD between 4 and 6mm) from 52 patients with CP (26 smokers/26 nonsmokers). Two test teeth and two control teeth were randomly selected from each patient (one tooth from each quadrant). CAL at 6 surfaces per tooth. Test sample size Baseline Test mean Baseline 52 teeth (26 people) smokers 6.7 Test Test SD or Mean Mean SD Test SD or SE sample Test mean at SE (list difference (or SE) (list value) size at end end of test value) at SD or SE? TEST (final- difference, Baseline of test period end of test baseline) TEST period period 1.1 52 teeth (26 people) smokers 6.4 1.1 SD Control sample size Baseline Control mean Baseline Control SD or SE (list value) Baseline 52 teeth (26 people) smokers 6.6 1.2 Control Control SD Control sample or SE (list mean at end size at end value) at SD or SE? of test on test end of test period period period 52 teeth (26 people) smokers 6.5 1.1 6.1 1.1 52 teeth (26 people) nonsmokers 5.6 1 SD Mean SD (or SE) Caries data difference, CONTROL SD Not reported 52 teeth (26 people) nonsmokers Mean difference CONTROL (finalbaseline) Not reported 52 teeth (26 people) nonsmokers 6.1 0.9 52 teeth (26 people) nonsmokers 5.8 1 No caries data Statstical analysis notes Only the changes in CAL for all groups proved to not be statistically significant at R1 and at R2 ( p > 0.05); no statistically significant changes between R1 and R2 were revealed by the intragroup analysis in all clinical parameters and GCF volume for all groups ( p > 0.05). SD Page 170 Nonsurgical use of lasers: Nd:YAG laser + SRP - Review authors’ judgment of each risk of bias item as low, unclear, or high for each included study Domain: Selection bias Random sequence Performance bias Allocation concealment Masking of participants Masking of personnel Were the groups Citation: Author, Year Support for judgment Review author's judgment Support for judgment Review author's judgment Support for judgment Review author's judgment Support for judgment Review author's judgment Support for judgment Eltas 2012 randomization claimed, but method not described Unclear No details given Unclear Not masked High Not masked High Yes Low Neill 1997 States random assignment but does not specify how Unclear OHI stated as given to control but not mentioned if given to other groups Unclear Unclear No details given Unclear No details given but "double blind" Unclear No details given but "double blind" Detection bias Attrition bias Reporting bias Masking of outcomes Incomplete outcome data Selective reporting Review Support for author's judgment judgment Review author's judgment Support for judgment Review author's judgment Support for judgment Review author's judgment Not involved in tx Low No patients lost Low No bias detected Low No details given but "double blind" Unclear No loss to follow up; data details sparse Unclear All data reported Low risk Nonsurgical use of lasers: Nd:YAG laser + SRP - Updated literature Selection bias Domain: Random sequence generation Performance bias Allocation concealment Masking of participants Masking of personnel Citation: Author, Year Support for judgment Review author's judgment Support for judgment Review author's judgment Support for judgment Eltas, 2012 coin toss Low Not described Unclear Placebo laser July 2015 Review Review Support for author's author's judgment judgment judgment Low Not described Unclear Were the groups treated the same except for the intervention? Detection bias Attrition bias Reporting bias Masking of outcomes assessment Incomplete outcome data (Include percent lost to follow-up) ?10% low; 11-20% unclear; >20% high Selective reporting Review Review Support for author's author's judgment judgment judgment Examiner No differences not involved Low Low noted in providing tx Support for judgment Support for judgment Review author's judgment Support for judgment Review author's judgment No losses Low None noted Low Page 171 Nonsurgical use of lasers: erbium laser + SRP - Study characteristics Citation: Author, Year Kelbauskiene 2011 Country Special population? (e.g. smokers) and other Severity of disease (e.g. data regarding inclusion refractory, mild, moderate) exclusion criteria for patients and teeth Lithuania Non-smokers only Early to moderate periodontitis Teeth exhibiting BOP, SRP with supragingival tooth subgingival calculus and PD of Single-rooted teeth (lower or upper Range 26 to 58 yrs Range 26 to 58 yrs cleaning 2 weeks prior and at 3 3-6mm on at least 1 site/tooth; incisors and canines, upper secon and 6 months single rooted teeth only. No premolars, lower first and second systemic antibiotics fopr prior premolars) 6 months Test subject Control subject age (mean, age (mean, median, range median, range as described) as described) Control Control Dose/ duration/ frequency/ timing if applicable Test (typically adjunct) Test (typically adjunct) Dose/ duration/ frequency/ timing including simultaneous/ before/ after Was standard Trial design counseling (split mouth / Duration of mentioned as part of parallel group / study control treatment? cross over) ultrasonic scaler and hand intstrumentation 9 mm Z6 tip 600 μm is set at 1 W, 10% air, 115% water is Er,Cr:YSGG laser used to remove junctional, sulcular and gingival epi 5mm from All subjects received OHI 2,790 nm margin; Laser tip angled 5-15 degrees toward root & moved and supragingival up a down until root appears etched. Procedure repeated cleaning 2 wks prior to tx plus SRP 1X/wk for each mm of pocket reduction required to achieve 3 mm depth Gracey curettes with local anesthesia until clean Er:YAG laser 2.94 μm, 250-500 μs exposure duration, 10Hz SRP+erbiumrepetition rate and a 1.1 X 0.5 mm tip: energy 100 mJ/pulse doped: yttrium, with transmitted energy of 71 mJ/pulse at the tip; fluency of aluminum,andgarn 12.9 J/cm2/pulse. Coolant water used and tip moved et (Er:YAG) laser apicocoronally at 30 degrees to the root. For the LA group irradiation irradiation was 180-240 seconds; for L+SRP 30 seconds/site after SRP Split mouth, 4 quads, one side SRP, other side SRP + laser Adverse events reported 12 months Not reported 12 months Not reported 6 months 5 perio abscesses reported, 3 in the prophy group and 2 in the laser + SRP group; 1 pt reported fever during the 1st week post-tx Non smokers Lopes 2010 Brazil Rotundo 2010 Italy sites with bleeding on probing (BOP) and probing depth (PD) from 5 to 9 mm were selected; no perio tx within previous 12 months; no systemic disease; no antibiotics prior 6 months; no anitinflammatories prior 3 months; not pregnant Moderate to severe chronic periodontitis 43 yrs (range 3155) 43 yrs (range 3155) Moderate to severe chronic periodontitis 50.5 ± 11.7 years 50.5 ± 11.7 years SRP OHI for 15-30 days and Split mouth, 1 site professional prophylaxis per quad 6 months pre-tx 12 of 27 smokers July 2015 2 teeth per quad with PD 4-9 mm with BOP; presence of at least one incisor, premolar and molar per quad Supragingival prophy SRP SRP with racey curettes and ultrasonic with local anesthesia PRN Laser + SRP Er:YAG laser 2.94 µm wavelength, energy level 150 mJ/pulse, repetition rate 10 Hz, fiber tip 0.5 mm and 10 mm length, coronal apical application at 20˚ inclination OHI Split mouth with quadrants randomly allocated to 2 controls and 2 treatments Page 172 Nonsurgical use of lasers: erbium laser + SRP - Outcomes data Citation: Author, Year Outcome measure Kelbauskiene 2011 CAL in the sites with baseline PD 3–6 mm; site averages given (n is listed by site) Time period Other time No. Sites treated for data periods for per mouth / No. presented in which data are sites averaged this available per tooth abstraction Test sample size Baseline Test mean Baseline Test Test SD or SE Test SD or SE sample Test mean at (list value) at (list value) size at end end of test end of test Baseline of test period period period SD or SE? Mean gain Control Mean SD TEST sample (or SE) (Baselinesize gain, TEST final) Baseline Control mean Baseline Control Control Control SD or Control SD or SE sample mean at end SE (list value) (list value) size at end of test at end of test Baseline on test period period period SD or SE? Mean gain Mean SD (or CONTROL SE) gain, (baselineCONTROL final) Caries data Statstical analysis notes NR Power analysis performed; Student's ttest, Kolmogorov-Smirnov test, MannWhainey U test; paired t-test for continuous data and Wilcoxon's test for ordinal data 30 pts Lopes 2010 CAL (computerized probe) 12 months 12 months 2,3,6 and 12 months 1, 3, 6 and 12 months 278 single rooted teeth; 9.3 teeth/pt; 1668 sites or 55.6 sites/pt 21 patients with four non-adjacent sites in different quadrants with bleeding on probing (BOP) and probing depth (PD) from 5 to 9 mm were selected 30 pts 135 teeth; 858 sites 21 30 pts 4.47 6.71 1.2 1.4 509 sites > 3 mm analyzed 19 patients; 76 sites in 76 teeth (n = 19 patients), 42 were at teeth with one or two roots and 34 were at multirooted teeth 2.8 1.27 SD 1.68 1.36 143 teeth or 8.4 teeth/pt 4.23 0.92 30 patients; 579 sites 1.3 19 patients; 76 sites in 76 teeth (n = 19 patients), 42 were at teeth with one or two roots and 34 were at multirooted teeth 3.4 1.19 SD 810 sites or 50.6/pt 5.56 1.4 SD Not reported Not reported 21 7.2 5.79 1.3 SD 0.84 1.09 Power calculation 95% with 19 pts based on CAL diff 1 mm; CAL values were normally distributed and analyzed using the analysis of variance for repeated measurements (ANOVA) test. The multiple comparison TukeyKramer test was used for comparison Not reported Not reported Not reported of PD and CAL variables among groups and periods when ANOVA test presented a significant difference (P <0.05) 1-mm clinical significant difference between groups, and a mean ± SD of 0.6 mm with the values of clinical attachment level (CAL). operator training; examiner calibration for intra examiner reliability Site = unit of analysis, ReML method for fitting mixed model, full factorial for Er:YAG laser and SRP, post-hoc Tukey-Kramer honestly significant difference test Rotundo 2010 July 2015 CAL 6 months 27 pts, all teeth with 1 week, 3 and 6 at least 1 site with PD months ≥ 4 mm; 6 sites/tooth 27 pts, 1671 sites ≥ 4 mm 26 pts, 1582 sites ≥ 4 mm L+SRP: 5.7 Laser + SRP grp 419 sites L+SRP: 1.5 Laser + SRP grp 405 sites L+SRP: 5.2 L+SRP: 1.8 27 pts, 1671 Not reported sites ≥ 4 mm (based on Not reported Not reported value, SRP grp 422 assumed SD) sites 26 pts, 1582 sites ≥ 4 mm SRP: 6.1 SRP: 1.6 SRP grp 399 sites SRP: 5.6 SRP: 2.0 The statistical analysis was intention to treat. In particular, if a patient showed up at the 3-month recall visit and not at the 6-month re-evaluation, Not reported the clinical data were imputed to the (based on Not reported Not reported Not reported 6-month analysis value, assumed SD) The sample size was calculated using a=0.05 and the power (1- b)=80%. For the variability (s=SD), the value of 0.6mm (Sculean et al. 2004) was used considering clinical attachment level (CAL) gain as a variable outcome. The minimum clinically significant value (d) considered was 0.5mm Page 173 Nonsurgical use of lasers: erbium laser + SRP - Review authors’ judgment of each risk of bias item as low, unclear, or high for each included study Domain: Selection bias Random sequence Performance bias Allocation concealment Masking of participants Masking of personnel Were the groups Citation: Author, Year Support for judgment Review author's judgment Support for judgment Review author's judgment Support for judgment Review author's judgment Support for judgment Review author's judgment Support for judgment Kelbauskiene 2011 Cards pulled from envelope Low No details given Unclear Not masked High Not masked High Yes Lopes 2010 Computer generated tables Low No details given Unclear Not masked High SRP clinician masked but laser person not High Rotundo 2011 Random assignement by quad, opaque sealed envelope sequentially number, computer generated random permuted block Low Envelope not opened utnil phase II tx Low No Unclear No details Unclear July 2015 Detection bias Attrition bias Reporting bias Masking of outcomes Incomplete outcome data Selective reporting Review Support for author's judgment judgment Review author's judgment Support for judgment Review author's judgment Support for judgment Review author's judgment Low No patients lost but some teeth surfaces lost that are unexplained Unclear Data from sites > 3 mm reported Low Low Only 1 site per tooth measured and reported; this was the planned approach Low Low All data reported Low Low Examiner kept blinded Yes Low Examiner did not know where laser treatment was used Low 19/21 finished although only out to 3 months for PICO question 1 Yes Low Examiner was always blinded as to treatment Low 24/26 completed to 6 months; 1 lost; 1 ITT only 3 month data Page 174 Appendix 4 – Details on data analysis calculations Combining results stratified by pocket depth If the data were only presented stratified by pocket depth, the data were combined by averaging the means, and for the standard deviation as follows: (∑𝑛 𝑆𝐷𝑖2 ) 𝑆𝐷𝑐𝑜𝑚𝑏𝑖𝑛𝑒𝑑 = √ 𝑖=1 𝑛 Data adjustments for meta-analysis calculations The equations used to compute the standard deviation (SD) and standard error (SE) between the treatment and control arms of individual trials are described below and differ by trial design (split-mouth versus parallel-group) and the type of data that were reported (baseline and followup data versus change scores). Correlation coefficients (designated as ) are required for the computations. These correlation coefficients refer to the correlation of results within the individual (for split-mouth trials) or over time (for baseline/follow-up trial reporting). (1) For split-mouth trials where the change score over time is given for both the treatment and control, the SD of the difference between treatment and control (effect) is calculated as: [SD( Difference )]2 sd12 sd22 2 sd1sd2 And then the standard error is calculated as: SE (mean difference ) SD(difference ) n The initial value of is set at 0.2560. Then, for sensitivity analysis, is changed to either -0.1 or 0.9, SDs and SEs recalculated for each trial, and a new meta-analysis performed to calculate the resulting overall summary estimate at different values of . (2) For split-mouth trials where the change score is not provided, the final values are used for treatment and control, and the protocol per (1) is conducted. There is a reference in the Cochrane Handbook that states this approach is statistically valid. July 2015 Page 175 (3) For parallel-group trials where no change score is provided, a process similar to (1) is used; however, the in this case represents the correlation with respect to time and sdBL and sdFINAL represents the baseline and final SDs for treatment and control: 𝑆𝐷𝑡𝑥 = √𝑠𝑑2𝑡𝑥,𝐵𝐿 + 𝑠𝑑2𝑡𝑥,𝐹𝑖𝑛𝑎𝑙 − 2𝜌𝑠𝑑𝑡𝑥,𝐵𝐿 𝑠𝑑𝑡𝑥,𝐹𝑖𝑛𝑎𝑙 𝑆𝐷𝑐 = √𝑠𝑑2𝑐,𝐵𝐿 + 𝑠𝑑2𝑐,𝐹𝑖𝑛𝑎𝑙 − 2𝜌𝑠𝑑𝑐,𝐵𝐿 𝑠𝑑𝑐,𝐹𝑖𝑛𝑎𝑙 Similar to split-mouth trials, the initial is set at 0.25, and then for the sensitivity analyses it is varied to -0.1 and 0.9. The equation for SE of the treatment vs. control difference (treatment effect) in this case is: 2 𝑆𝐸(𝑀𝑒𝑎𝑛 𝐷𝑖𝑓𝑓𝑒𝑟𝑒𝑛𝑐𝑒) = √𝑆𝐷𝑡𝑥 /𝑛𝑡𝑥 + 𝑆𝐷𝑐2 /𝑛𝑐 And the sensitivity analyses are conducted using the previously calculated SDs. (4) For parallel-group trials where the change score is provided, then no correlation coefficients are needed (and no adjustments for sensitivity analysis) and only the SE(Mean Difference) needs to be calculated per (3): 2 𝑆𝐸(𝑀𝑒𝑎𝑛 𝐷𝑖𝑓𝑓𝑒𝑟𝑒𝑛𝑐𝑒) = √𝑆𝐷𝑡𝑥 /𝑛𝑡𝑥 + 𝑆𝐷𝑐2 /𝑛𝑐 The standard generic inverse-variance methods are then used to calculate the pooled effect estimate. July 2015 Page 176 Specific methods for standard error imputation when data not provided SRP section Kahl study: Pg. 320 states, ‘Initial shallow pockets (<0.3mm), no changes in PD and AL were found; these results are therefore not shown.’ From Fig. 1 we have 6% of values are deep pockets, but only 24% are moderate. The remaining 70% are shallow pockets with no difference between groups are not reported. However, as the intervention essentially has no effect on healthy sites, 6% deep sites and 24% moderate sites can be assumed to represent all sites. Therefore, there is a 1/4 ratio of deep to moderate sites leading to 25% deep and 75% moderate sites. It is reported in the paper and from Fig. 5 and 6 that there is a 0.6mm CAL gain in moderate sites and a 0.9mm CAL gain in deep sites. Therefore, MD of 0.25 (0.9) + 0.75 (0.6) = 0.675 mm. Note, that if the shallow sites were counted as representing 70% of the sites, the MD would drop down to 0.20mm. The following SD and SE calculations were based on a 25/75 split between deep and shallow pockets as well. sd(bl) = 0.75 (1.5) + 0.25 (1.4) = 1.475 sd(final) = 0.75 (1.5) + 0.25 (1.2) = 1.425 SD(diff) = sqrt 1.482 + 1.432 - 2 (0.25)(1.48)(1.43) SD(diff) = 1.78 SE = 1.78 / sqrt(20) SE = 0.39 if correlation coefficient is ignored, then the SE jumps slightly to 0.46; if correlation coefficient is -0.1 then SE = 0.48; if correlation coefficient is 0.9 then SE = 0.15; 2. Lindhe study: using Kahl data: SE = 1.78 / sqrt(7) SE = 0.67 for correlation coefficient of 0.25 SE = 0.82 for correlation coefficient of -0.1 SE = 0.25 for correlation coefficient of 0.9 3. Berglundh study (note also in systemic antimicrobials section): using Kahl data: SE = 1.78 / sqrt (8) SE = 0.63 for correlation coefficient of 0.25 July 2015 Page 177 SE = 0.76 for correlation coefficient of -0.1 SE = 0.23 for correlation coefficient of 0.9 4. Ng study (note: in both SRP and systemic antimicrobials section): SD = 0.83 SE = 0.29 for correlation coefficient 0.25 SE = 0.35 for correlation coefficient -0.1 SE = 0.15 for correlation coefficient 0.9 Lasers – PDT section Gianelli study: SE= 0.2 for mean difference at one year follow-up with no correlation coefficient adjustments. Systemic Antimicrobial section 1. Mombelli study: Used data from Table 2 rather than Figure 2, and went under assumption that SD, rather than SE were given. This does not coincide perfectly with Table 1 baseline data, but it’s the best data provided in the study; thus: SD (tx) = sqrt 2.72 + 2.42 - 2(0.25)(2.7)(2.4) SD (tx) = sqrt 7.29 + 5.76 - 3.24 SD (tx) = 3.13 SD (c) = sqrt 2.32 + 1.62 - 2(0.25)(2.3)(1.6) SD (c) = sqrt 5.29 + 2.56 - 1.84 SD (c) = 2.45 therefore SE (mean difference) = sqrt 3.132 / 7 + 2.452 / 7 SE (mean difference) = sqrt 1.40 + 0.86 SE (mean difference) for correlation coefficient 0.25 = 1.50 SE (mean difference) for correlation coefficient -0.10 = 1.70 SE (mean difference) for correlation coefficient 0.90 = 0.61 July 2015 Page 178 MD = 1.8 from this method. 2. Ng study: Impute data as we have no SD change scores. SD = 0.83 SE = 0.29 for correlation coefficient 0.25 SE = 0.35 for correlation coefficient -0.1 SE = 0.15 for correlation coefficient 0.9 3. Pradeep Study: Use 9-month data even though SRP repeated at 6 months: MD = 1.07. 4. Berglundh study (note also in SRP section): using Kahl data: SE = 1.78 / sqrt (8) SE = 0.63 for correlation cofficient of 0.25 SE = 0.76 for correlation cofficient of -0.1 SE = 0.23 for correlation cofficient of 0.9 5. Lindhe study (note also in SRP section): using Kahl data: SE = 1.78 / sqrt(7) SE = 0.67 for correlation cofficient of 0.25 SE = 0.82 for correlation cofficient of -0.1 SE = 0.25 for correlation cofficient of 0.9 July 2015 Page 179