* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Thermodynamics
Electrochemistry wikipedia , lookup
Thermal radiation wikipedia , lookup
Van der Waals equation wikipedia , lookup
Marcus theory wikipedia , lookup
George S. Hammond wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Heat transfer physics wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
Glass transition wikipedia , lookup
Thermal conduction wikipedia , lookup
Temperature wikipedia , lookup
Thermodynamics wikipedia , lookup
Thermal expansion wikipedia , lookup
Equation of state wikipedia , lookup
Thermoregulation wikipedia , lookup
Transition state theory wikipedia , lookup
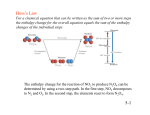
Thermodynamics Intensive and extensive properties • Intensive properties: – System properties whose magnitudes are independent of the total amount, instead, they are dependent on the concentration of substances • Extensive properties – Properties whose value depends on the amount of substance present State and Nonstate Functions • Euler’s Criterion • State functions – Pressure – Internal energy • Nonstate functions – Work – Heat Energy • Capacity to do work • Internal energy is the sum of the total various kinetic and potential energy distributions in a system. Heat • The energy transferred between one object and another due to a difference in temperature. • In a molecular viewpoint, heating is: – The transfer of energy that makes use of disorderly molecular motion • Thermal motion – The disorderly motion of molecules Exothermic and endothermic • Exothermic process – A process that releases heat into its surroundings • Endothermic process – A process wherein energy is acquired from its surroundings as heat. Work • Motion against an opposing force. • The product of an intensity factor (pressure, force, etc) and a capacity factor (distance, electrical charge, etc) • In a molecular viewpoint, work is: – The transfer of energy that makes use of organized motion Free expansion Isothermal ΔU 0 q nRT ln 𝑉𝑓 Isochoric Isobaric q+w 𝑉 𝑖 wrev wirrev 0 𝑉 -nRT ln 𝑉𝑓 0 𝑖 -pextΔV -pextΔV Adiabatic Adiabatic Changes • q=0! • Therefore ΔU = w • In adiabatic changes, we can expect the temperature to change. • Adiabatic changes can be expressed in terms of two steps: the change in volume at constant temperature, followed by a change in temperature at constant volume. Adiabatic changes • The overall change in internal energy of the gas only depends on the second step since internal energy is dependent on the temperature. • ΔUad = wad = nCvΔT for irreversible conditions Adiabatic changes • How to relate P, V, and T? during adiabatic changes? Use the following equations! • ViTic = VfTfc – Where c = Cv/R • PiViγ = PfVfγ – Where γ = Cp/Cv Adiabatic changes • What about for reversible work? • Wad,rev = 𝑛𝑅∆𝑇 1−γ Free Isothermal expansion ΔU 0 q nRT ln 𝑓 𝑉𝑖 or -wirrev Isochoric Isobaric Adiabatic nCvΔT q+w w nCvΔT nCpΔT or –wirrev 0 𝑉 wrev 0 wirrev 0 -nRT ln 𝑉𝑓 𝑉𝑖 -pextΔV 0 0 -nRT ln 𝑉𝑓 𝑉𝑖 -pextΔV −𝑛𝑅∆𝑇 1−γ =-nCvΔT =-pextΔV Exercise 1. 2. 10 g of N2 is obtained at 17°C under 2 atm. Calculate ΔU, q, and w for the following processes of this gas, assuming it behaves ideally: (5 pts each) a) Reversible expansion to 10 L under 2 atm b) Adiabatic free expansion c) Isothermal, reversible, compression to 2 L d) Isobaric, isothermal, irreversible expansion to 0.015 m3 under 2 atm e) Isothermal free expansion (Homework) 2 moles of a certain ideal gas is allowed to expand adiabatically and reversibly to 5 atm pressure from an initial state of 20°C and 15 atm. What will be the final temperature and volume of the gas? What is the change in internal energy during this process? Assume a Cp of 8.58 cal/mole K (10 pts) 1 cal = 4.184 J Enthalpy • As can be seen in the previous derivation, at constant pressure: • ΔH = nCpΔT Relating ΔH and ΔU in a reaction that produces or consumes gas • ΔH = ΔU + pΔV, • When a reaction produces or consumes gas, the change in volume is essentially the volume of gas produced or consumed. • pΔV = ΔngRT, assuming constant temperature during the reaction • Therefore: › ΔH = ΔU + ΔngRT Dependence of Enthalpy on Temperature • The variation of the enthalpy of a substance with temperature can sometimes be ignored under certain conditions or assumptions, such as when the temperature difference is small. • However, most substances in real life have enthalpies that change with the temperature. • When it is necessary to account for this variation, an approximate empirical expression can be utilized Dependence of Enthalpy on Temperature 𝑑𝐻 = 𝐶𝑝𝑑𝑡 𝐻 (𝑇𝑓) 𝑇𝑓 𝑑𝐻 = 𝐻 (𝑇𝑖) • Where the empirical parameters a, b, and c are independent of temperature and are specific for each substance 𝑇𝑖 𝐶𝑝 𝑑𝑡 • Integrate resulting equation for Cp appropriately in order to get ΔH Free expansion Isothermal Isochoric Isobaric Adiabatic ΔU 0 nCvΔT q+w w q nRT ln 𝑓 𝑉𝑖 or -wirrev nCvΔT nCpΔT or – wirrev 0 𝑉 wrev 0 wirrev 0 ΔH -nRT ln 𝑉𝑓 𝑉𝑖 0 -nRT ln 𝑉𝑓 𝑉𝑖 −𝑛𝑅∆𝑇 1−γ -pextΔV 0 -pextΔV =-nCvΔT =-pextΔV 0 (for ideal gas) ΔU =ΔU + pΔV =nCpΔT Adiabatic Problem • Calculate the change in molar enthalpy of N2 when it is heated from 25°C to 100°C. N2(g) Cp,m (J/mol K) a =28.58; b = 3.77x10-3 K; c = -0.50 x105 K2 Problem • Water is heated to boiling under a pressure of 1.0 atm. When an electric current of 0.50 A from a 12V supply is passed for 300 s through a resistance in thermal contact with it, it is found that 0.798 g of water is vaporized. Calculate the molar internal energy and enthalpy changes at the boiling point. *1AVs=1J Thermochemistry • The study of energy transfer as heat during chemical reactions. • This is where endothermic and exothermic reactions come in. • Standard enthalpy changes of various kinds of reactions have already been determined and tabulated. Standard Enthalpy Changes • ΔHƟ • Defined as the change in enthalpy for a process wherein the initial and final substances are in their standard states – The standard state of a substance at a specified temperature is its pure form at 1 bar • Standard enthalpy changes are taken to be isothermal changes, except in some cases to be discussed later. Enthalpies of Physical Change • The standard enthalpy change that accompanies a change of physical state is called the standard enthalpy of transition • Examples: standard enthalpy of vaporization (ΔvapHƟ) and the standard enthalpy of fusion (ΔfusHƟ) Enthalpies of chemical change • These are enthalpy changes that accompany chemical reactions. • We utilize a thermochemical equation for such enthalpies, a combination of a chemical equation and the corresponding change in standard enthalpy. • Where ΔHƟ is the change in enthalpy when the reactants in their standard states change to the products in their standard states. Hess’s Law • Standard enthalpies of individual reactions can be combined to acquire the enthalpy of another reaction. This is an application of the First Law named the Hess’s Law • “The standard enthalpy of an overall reaction is the sum of the standard enthalpies of the individual reactions into which a reaction may be divided.” Hess’s Law: Example Standard Enthalpies of Formation • The standard enthalpy of formation, denoted as ΔfHƟ, is the standard reaction enthalpy for the formation of 1 mole of the compound from its elements in their reference states. • The reference state of an element is its most stable state at the specified temperature and 1 bar. • Example: Benzene formation 6 C (s, graphite) + 3 H2(g) C6H6 (l) ΔfHƟ = 49 kJ/mol The temperature dependence of reaction enthalpies • dH = CpdT • From this equation, when a substance is heated from T1 to T2, its enthalpy changes from the enthalpy at T1 to the enthalpy at T2. Kirchhoff’s Law • It is normally a good approximation to assume that ΔrCpƟ is independent of temperature over a limited temperature range, but when the temperature dependence of heat capacities must be taken into account, we can utilize another equation Dependence of Enthalpy on Temperature 𝑑𝐻 = 𝐶𝑝𝑑𝑡 𝐻 (𝑇𝑓) 𝑇𝑓 𝑑𝐻 = 𝐻 (𝑇𝑖) • Where the empirical parameters a, b, and c are independent of temperature and are specific for each substance 𝑇𝑖 𝐶𝑝 𝑑𝑡 • Integrate resulting equation for Cp appropriately in order to get ΔH Kirchhoff’s Law: Example Exercise