* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 10/3/16 Intro Atomic Theories
Survey
Document related concepts
Transcript
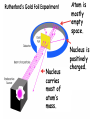
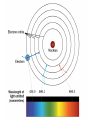
QQ: 1. Atom B has a mass of 42 and an atomic number of 20. How many protons, electrons, and neutrons does it have? 2. Atom K has 15 electrons and 10 neutrons. What is its atomic number and atomic mass? 3. An atom with 64 protons has an atomic mass of 64. What is its atomic number? How many electrons and neutrons does it have? Today’s Objective: I understand the development of current atomic theory. Vocabulary Quiz Is it possible to figure out what this is without unwrapping it? Follow instructions carefully. Do not remove any papers or look underneath Figure out the phrase. Write down a guess. Remove the top sheet. Write down your new prediction. How did the information on the sheets change as you moved through the stack? How did the number of letters provided affect your ability to predict the phrase? Describe another situation in which having more data makes it easier to make a prediction. Democritus 400 BC Unable to be divided Aristotle 400 BC • • • • 4 basic elements Air Earth Fire Water Dalton 1776 Dalton 1803 Same mass Can make compounds Thomson 1897 Subatomic particles: electron Thomson: Plum Pudding Rutherford 1911 Nucleus has + charge Rutherford: Gold Foil Experiment Nucleus is Positive Most is Atom particles mostly traveled empty through space. the foil. Some is Nucleus particles positively were charged. Nucleus Occasionally reflected carries bounced back. most back. of atom’s mass. Bohr 1913 More energy in levels Farther away from nucleus Electron Configurations: Arrangements of electrons around the nucleus based on their energy Level. Electron Cloud Theory Schrodinger 1926 Cloud shows areas where electrons are likely to be found. Schrodinger's Cat A Weird and Wondrous Place Q2: Work on your news article. Remember: • Newspaper format • Scientist & His Discovery • Year of Discovery • Previous Theories • Picture • Quality of Product Draw and complete the table. Draw and complete the table. 7 5 9 19 5 18 18 29 7 19 18 6