* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Solid State Synthesis
Survey
Document related concepts
Transcript
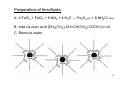
Solid State Synthesis • • • • Solid State Reactions Film deposition Sol-gel method Crystal Growth 1 Synthesis References • A.R. West "Solid State Chemistry and its Applications" Chapter 2 – Preparative Methods • "Solid-State Chemistry – Techniques" Chapter 1 – Synthesis of Solid-State Materials J.D. Corbett – book edited by A.K. Cheetham and P. Day • "Preparation of Thin Films" Joy George This book has a nice succinct treatment of the various thin film deposition methods. The following references discuss various aspects or methods in solid state synthesis in greater detail. Low Temperature & Precursor Techniques • "Crystallization of Solid State Materials via Decomplexation of Soluble Complexes" K.M. Doxsee, Chem. Mater. 10, 2610-2618 (1998). • "Accelerating the kinetics of low-temperature inorganic syntheses" R.Roy J. Solid State Chem. 111, 11-17 (1994). • "Nonhydrolytic sol-gel routes to oxides" A. Vioux, Chem. Mater. 9, 2292-2299 (1997). Molten Salt Fluxes & Hydrothermal Synthesis • "Turning down the heat: Design and mechanism in solid state synthesis" 2 A. Stein, S. W. Keller, T.E. Mallouk, Science 259, 1558-1563 (1993). • • "Synthesis and characterization of a series of quaternary chalcogenides BaLnMQ3 (Ln = rare earth, M = coinage metal, Q = Se or Te)" Y.T. Yang, J.A. Ibers, J. Solid State Chem. 147, 366-371 (1999). "Hydrothermal Synthesis of Transition metal oxides under mild conditions" M.S. Whittingham, Current opinion in Solid State & Materials Science 1, 227232 Chimie Douce & Low Temperature Synthesis • "Chimie Douce Approaches to the Synthesis of Metastable Oxide Materials" J. Gopalakrishnan, Chem. Mater. 7, 1265-1275 (1995). High Pressure Synthesis • "High pressure synthesis of solids" P.F. McMillan, Current Opinion in Solid State & Materials Science 4, 171-178 (1999) • "High-Pressure Synthesis of Homologous Series of High Cricitcal Temperature (Tc) Superconductors" E. Takayama-Muromachi, Chem. Mater. 10, 2686-2698 (1998). • "Preparative Methods in Solid State Chemistry" J.B. Goodenough, J.A. Kafalas, J.M. Longo, (edited by P. Hagenmuller) Academic Press, New York (1972). 3 Classification of Solids There are several forms solid state materials can adapt Single Crystal Preferred for characterization of structure and properties. Polycrystalline Powder (Highly crystalline) Used for characterization when single crystal cannot be easily obtained, preferred for industrial production and certain applications. Polycrystalline Powder (Large Surface Area) Desirable for further reactivity and certain applications such as catalysis and electrode materials Amorphous (Glass) No long range translational order. Thin Film Widespread use in microelectronics, telecommunications, optical applications, coatings, etc. 4 Solid State Reactions (1) Area of contact between reacting solids - We want to use starting reagents with large surface area to maximize the contact between reactants Consider the numbers for a 1 cm3 volume of a reactant • Edge Length = 1 cm # of Crystallites = 1 Surface Area = 6 cm2 • Edge Length = 10 μm # of Crystallites = 109 Surface Area = 6 x 103 cm2 • Edge Length = 100Å # of Crystallites = 1018 Surface Area = 6 x 106 cm2 - Pelletize to encourage intimate contact between crystallites. 5 Time (h) Schematic reaction, by interdiffusion of cations of single crystals of MgO and Al2O3, (c) Thickness, x of MgAl2O3 product layer as a function of temperature and time. (Pettit, Randklev and Felton, 1966) 6 Different parts of the crystal have different structures and different reactivities 7 (2) The rate of diffusion Two ways to increase the rate of diffusion • Increase temperature • Introduce defects by starting with reagents that decompose prior to or during reaction, such as carbonates or nitrates. 8 9 (3) The rate of nucleation of the product phase • Maximize the rate of nucleation by using reactants with crystal structures similar to that of the product (topotactic and epitactic reactions). a topotactic transformation is characterized by internal atomic displacements, which may include loss or gain of material so that the initial and final lattices are in coherence. epitaxy - The growth of the crystals of one mineral on the crystal face of another mineral, such that the crystalline substrates of both minerals have the same structural orientation. 10 What are the consequences of high reaction temperatures? • It can be difficult to incorporate ions that readily form volatile species (i.e. Ag+). • It is not possible to access low temperature, metastable (kinetically stabilized) products. • High (cation) oxidation states are often unstable at high temperature, due to the thermodynamics of the following reaction: 2MOn (s) Æ 2MOn-1(s) + O2(g) Due to the release of a gaseous product (O2), the products are favored by entropy, and the entropy contribution to the free energy become increasingly important as the temperature increases. 11 Steps in Conventional Solid State Synthesis 1). Select appropriate starting materials a) Fine grain powders to maximize surface area b) Reactive starting reagents are better than inert c) Well defined compositions 2). Weigh out starting materials 3). Mix starting materials together a) Agate mortar and pestle (organic solvent optional) b) Ball Mill (Especially for large preps > 20g) 4). Pelletize 12 Steps in Conventional Solid State Synthesis (continued) 5). Select sample container Reactivity, strength, cost, ductility are all important a) Ceramic refractories (crucibles and boats) Al2O3 1950 °C $30/(20 ml) ZrO2/Y2O3 2000 °C $94/(10 ml) b) Precious Metals (crucibles, boats and tubes) Pt 1770 °C $500/(10 ml) Au 1063 °C $340/(10 ml) c) Sealed Tubes SiO2- Quartz, Au, Ag, Pt 13 6)Heat a) Factors influencing choice of temperature for volatilization b) Initial heating cycle to lower temperature can help to prevent spillage and volatilization c) Atmosphere is also critical Oxides (Oxidizing Conditions) – Air, O2, Low Temps Oxides (Reducing Conditions) – H2/Ar, CO/CO2, High T Nitrides – NH3 or Inert (N2, Ar, etc.) Sulfides – H2S Sealed tube reactions, Vacuum furnaces 7) Grind product and analyze (x-ray powder diffraction) 8) If reaction incomplete, return to step 4 and repeat. 14 Example: the synthesis of Sr2CrTaO6 1) Possible starting reagents Sr Metal – Hard to handle, prone to oxidation SrO - Picks up CO2 & water, mp = 2430 °C Sr(NO3)2 – mp = 570 °C, may pick up some water SrCO3 – decomposes to SrO at 1370 °C Ta Metal – mp = 2996 °C Ta2O5 – mp = 1800 °C Cr Metal – Hard to handle, prone to oxidation Cr2O3 – mp = 2435 °C Cr(NO3)3*nH2O – mp = 60 °C, composition inaccurate 15 • To make 5.04 g of Sr2CrTaO6 (FW = 504.2 g/mol; 0.01 mol) • complete the reaction: 4SrCO3 + Ta2O5 + Cr2O3 Æ 2Sr2CrTaO6 + 4CO2 • you need: SrCO3 2.9526 g (0.02 mol) Ta2O5 2.2095 g (0.005 mol) Cr2O3 0.7600 g (0.005 mol) 16 • Applying Tamman’s rule to each of the reagents: SrCO3 ⇒ SrO 1370 °C (1643 K) SrO mp = 2700 K ® 2/3 mp = 1527 °C Ta2O5 mp = 2070 K ® 2/3 mp = 1107 °C Cr2O3 mp = 2710 K ® 2/3 mp = 1532 °C • Although you may get a complete reaction by heating to 1150 °C, in practice there will still be a fair amount of unreacted Cr2O3. Therefore, to obtain a complete reaction it is best to heat to 1500-1600 °C. 17 Precursor Routes • • • Approach : Decrease diffusion distances through intimate mixing of cations. Advantages : Lower reaction temps, possibly stabilize metastable phases, eliminate intermediate impurity phases, produce products with small crystallites/high surface area. Disadvantages : Reagents are more difficult to work with, can be hard to control exact stoichiometry in certain cases, sometimes it is not possible to find compatible reagents (for example ions such as Ta5+ and Nb5+ immediately hydrolyze and precipitate in aqueous solution). 18 Precursor Routes (continued) • • Methods : With the exception of using mixed cation reactants, all precursor routes involve the following steps: 1. Mixing the starting reagents together in solution. 2. Removal of the solvent, leaving behind an amorphous or nano-crystalline mixture of cations and one or more of the following anions: acetate, citrate, hydroxide, oxalate, alkoxide, etc. 3. Heat the resulting gel or powder to induce reaction to the desired product. The following case studies illustrate some examples of actual syntheses carried out using precursor routes. 19 Coprecipitation Synthesis of ZnFe2O4 • Mix the oxalates of zinc and iron together in water in a 1:1 ratio. Heat to evaporate off the water. As the amount of H2O decreases, a mixed Zn/Fe oxalate (probably hydrated) precipitates out. Fe2 ((COO) 2) 3 + Zn(COO) 2Æ Fe2Zn((COO) 2) 5*xH2O • After most of the water is gone, the precipitate is filtered and calcined at 1000 °C. Fe2Zn((COO) 2) 5Æ ZnFe2O4 + 4CO + 4CO2 20 Precursor Routes (continued) • This method is easy and effective when it works. It is not suitable when Reactants of comparable water solubility cannot be found. The precipitation rates of the reactants are markedly different. These limitations make this route unpractical for many combinations of ions. Furthermore, accurate stoichiometric ratios may not always be maintained. 21 Molten Salt Fluxes • Solubilize reactants → Enhance diffusion → Reduce reaction temperature • Synthesis in a solvent is the common approach to synthesis of organic and organometallic compounds. This approach is not extensively used in solid state syntheses, because many inorganic solids are not soluble in water or organic solvents. However, molten salts turn out to be good solvents for many ioniccovalent extended solids. • Often slow cooling of the melt is done to grow crystals, however if the flux is water soluble and the product is not then powders can also be made in this way and separated from the excess flux by washing with water. 22 Molten Salt Fluxes (continued) • Synthesis needs to be carried out at a temperature where the flux is a liquid. • Purity problems can arise, due to incorporation of the molten salt ions in product. This can be overcome either by using a salt containing cations and/or anions which are also present in the desired product (i.e. synthesis of Sr2AlTaO6 in a SrCl2 flux) , or by using salts where the ions are of a much different size than the ions in the desired product (i.e. synthesis of PbZrO3 in a B2O3 flux). 23 Example 1 • 4SrCO3 + Al2O3 + Ta2O5 ÆSr2AlTaO6 (SrCl2 flux, 900°C) • Powder sample, wash away SrCl2 with weakly acidic H2O • Direct synthesis requires T > 1400°C and Sr2Ta2O7 impurities persist even at 1600° C 24 25 26 Solid State Metathesis Reactions A metathesis reaction between two salts merely involves an exchange of anions, although in the context we will use there can also be a redox component. If the appropriate starting materials are chosen, a highly exothermic reaction can be devised. MoCl5 + 5/2 Na2S ÆMoS2 + 5NaCl + ½ S The enthalpy of this reaction is ∆H = -213 kcal/mol 27 28 Hydrothermal Synthesis • Reaction takes place in superheated water, in a closed reaction vessel called a hydrothermal bomb (150 < T < 500 °C; 100 < P < 3000 kbar). • Seed crystals and a temperature gradient can be used for growing crystals • Particularly common approach to synthesis of zeolites • Example : 6CaO + 6SiO2 Æ Ca6Si6O17(OH)2 (150-350 °C) 29 30 31 Sol-gel process 32 33 34 35 36 Intercalation • Involves inserting ions into an existing structure, this leads to a reduction (cations inserted) or an oxidation (anions inserted) of the host. • Typically carried out on layered materials (strong covalent bonding within layers, weak van der Waals type bonding between layers, i.e. graphite, clays, dicalchogenides,). • Performed via electrochemistry or via chemical reagents as in the n-butyl Li technique. Examples : TiS2 + nBu-Li Æ LiTiS2 b-ZrNCl + Naph-Li Æ b-LixZrNCl 37 38 39 40 Dehydration • By removing water and/or hydroxide groups from a compound, you can often perform redox chemistry and maintain a structural framework not accessible using conventional synthesis approaches • Examples : Ti4O7(OH)2*nH2O Æ TiO2 (B) (500° C) 2KTi4O8(OH)*nH2O Æ K2Ti8O17 (500° C) 41 Ion Exchange • Exchange charge compensating, ionically bonded cations (easiest for monovalent cations) • Examples : LiNbWO6 + H3O + Æ HNbWO6 + Li+ KSbO3 + Na + Æ NaSbO3 + K + 42 Film Formation • Dip Coating • Spin Coating • Vapor Deposition • Chemical Vapor Deposition 43 Chemical vapor deposition (CVD) is a chemical process for depositing thin films of various materials. In a typical CVD process, the substrate is exposed to one or more volatile precursors, which react and/or decompose on the substrate surface to produce the desired deposit. Frequently, volatile by-products are also produced, which are removed by gas flow through the reaction chamber. 44 Types of CVD Processes • • • • • • • • Atmospheric Pressure Chemical Vapour Deposition (APCVD) Low Pressure Chemical Vapour Deposition (LPCVD) Metal-Organic Chemical Vapour Deposition (MOCVD) Plasma Assisted Chemical Vapour Deposition (PACVD) or Plasma Enhanced Chemical Vapour Deposition (PECVD) Laser Chemical Vapour Deposition (LCVD) Photochemical Vapour Deposition (PCVD) Chemical Vapour Infiltration (CVI) Chemical Beam Epitaxy (CBE) 45 Film formation (a) (b) 46 MOCVD to prepare METAL, METAL OXIDE, NITRIDE and SULFIDE FILMS Metal-organic CVD (MOCVD) - CVD processes based on metal-organic precursors, such as Tantalum Ethoxide, Ta(OC2H5)5, to create TaO, Tetra Dimethyl amino Titanium (or TDMAT) to create TiN. The philosophy behind this work is the discovery of: • volatile organometallic precursors • sometimes single source containing more than one of the required elements • that are pure enough • and cleanly produce the required elements on a desired substrate • at as low a temperature as possible • often epitaxially to minimize interfacial defects 47 MOCVD PRECURSORS A favorite ligand is the bulky 2,2',6,6'-tetramethyl-3,5heptanedionate, basically a bulky acac ligand (TMHD) Y(TMHD)3 Tsub = 160oC, Ba(TMHD)2 Tsub = 70-190oC, and Cu(TMHD)2 Tsub = 125oC are very useful MOCVD precursors 48 MOCVD to prepare METAL, METAL OXIDE, NITRIDE and SULFIDE FILMS • Best precursors for copper films used in microelectronics are Cu(hfacac)2 (VP 0.25 Torr at 60oC) at 250-350oC, and Cu(hfacac)PR3 (VP 0.1 Torr at 60oC) at 120-350oC hfacac = hexafluoroacetylacetonate • Rare earth doped semiconductor films make use of the sterically crowded encapsulated (C5H4Me)3Nd and (C5H4CMe3)3Nd can sublime at 110oC and 10-3 Torr allowing them to be doped into III-V semiconductors, the idea is to excite the sharp 4f-4f intra-shell luminescence of the rare earth center optically and electrically via the host semiconductor crystals, which is of interest in fiber optical communication: GaMe3 + AsH3 + (C5H4Me)3Nd ⇒ Nd:GaAs 49 • Nitride films are important as they display unique properties including metallic behavior, extreme hardness, very high melting points, high chemical resistance This has generated considerable interest in MOCVD precursors to nitride films Homoleptic dialkyamides and ammonia react at temperatures as low as 200oC to afford excellent quality TiN films: Ti(NMe2)4 + NH3 (200-450oC) ⇒ TiN + organics • Sulfide films possess a wide range of fascinating solid state properties and have been the focus of much MOCVD research. Most prominent application is in the area of cathodes for thin film lithium batteries. Promising materials are TiS2 and MoS2 TiCl4(HSC6H11)2 (VP 1-2 Torr, 25oC, single source precursor, ~200oC) ⇒ TiS2 + 2HCl + 2C6H11Cl 50 Ceramic Materials 51 Ceramic parts made of Si3N4 Thermal Insulating Ceramic Tiles for space shuttle 52 53 54 CVD to produce diamond at low pressure and around 1000oC Single crystal synthetic diamonds (3000oC and 130 kbar from graphite) make excellent heat sinks for semiconductors in device applications Example of high thermal conductivity of diamond laser diode heat drain (yellow diamond n-doped with N, blue diamond p-doped with B), conductivity of diamond 4x greater than copper or silver at RT (10-20 watts/cmoC) By 1996, it was estimated that semiconductor applications could take 60% of worldwide diamond thin film market, other contenders for use of diamond film made by CVD are coated tools (abrasion resistance), optical disk coatings (protective coatings), lens and window coatings, loudspeakers (sound distortion control), UV laser coatings (reduces laser heating) 55 Synthetic methods for making diamond films all employ low pressure deposition of 1%CH4/H2 onto 1000oC substrate • Heated filament method uses a hot wire to decompose the methane, 2200oC, produces atomic C/H, 50 Torr pressure silica bell jar, diamond film deposited on 1000oC substrate • Direct current plasma jet arc discharge focuses coating on a small area of substrate and can be scanned across a substrate • Microwave plasma discharge is used for commercial production of diamond films 56 Highly Organized Carbon Nanotube by Chemical Vapor Deposition B. Q. Wei, R. Vajtai, Y. Jung, J. Ward, R. Zhang, G. Ramanath, P. M. Ajayan xylene + [Fe(C5H5)2] with flowing argon to 100mTorr and heated gradually to 800 °C 57 58 59 60 61 62 Nano Materials Nanotechnology comprises any technological developments on the nanometer scale, usually 0.1 to 100 nm. Synthesis of Nano Materials 1. Chemical processes 2. Physical processes <Ref>: Nanomaterials: Synthesis, Properties & Applications, eds. A.S. Edelstein & R.C. Cammarata 63 Metal particle size effect atom → clusters → bulk metallic particle • geometry • coordination number • chemical properties change a lot for particles ≤ 55 atoms ~ 1 nm diameter • Particle size effect, usually investigated in the range of 10 ~ 50 Å in diameter. • When > 40-50 Å, the crystals exhibit “bulk” behavior <ref> Adv. Catal. 36, 55 (1989) 64 Lowering of melting point on Nano-metal Particles Size Effect on Melting Point gold: 1063°C → 330°C (2nm) silver: 960°C → 100°C (2nm) boiling water can melt silver Goldstrin et al, Science (1992) 65 Electronic effect Electronic effect only apparent for particles < 2nm 66 67 Unit Cells In Cubic Crystal Structures simple cubic Body-centered cubic (primitive cubic) (bcc) Face-centered cubic (fcc) 68 C9 C7 C8 C8 C7 C6 69 Geometric effect terrace edge corner 70 # atoms along the particle edge Coordination # 71 72 73 Particle Size Effect body-center cubic (bcc) Fe N 2 + H 2 ⎯⎯→ NH 3 Dumestic et al. Larger particles are more active than small ones because their surface has a higher fraction of 7-coord. Fe atoms. Spencer et al. at 525℃, 200 atm rate on (111):(100):(110)=418:25:1. body center cubic (111) poses a large conc. of C7. 74 Monodispersed metal oxide particles prepared from homogeneous solutions Preparation routes : 1. Deprotonation of hydrated cations 2. Controlled release of precipitating anions 3. Thermal decomposition of metal complexes, such as organometallic compounds 75 Preparation and Properties of Monodispersed Colloidal Particles of Lanthanide Compounds. Langmuir, 4, (1988) 32 76 Size distribution analysis : A comparison of the histogram obtained by electron microscopy with the distributions from light scattering at two wavelengths (436 and 546 nm). 77 Precipitation domain for solutions containing Ce(S04)2 and H2SO4 aged at 90 "C for 12 h. Symbols designating different kinds of particles: ○, spheres; □, rods, , rods mixed with spheres; , a very small amount of spheres; x, no particle formation. 78 79 Chemical processes for preparing nano-particles 80 81 Nucleation and Growth of CdSe on ZnS Quantum Crystallite Seeds, and Vice Versa, in Inverse Micelle Media J. Am. Chem. SOC., 112 (1990) 1332 Figure 7. Bright field transmission electron micrograph of (ZnS),-(CdSe),Ph particles. Particles showing lattice images have ( 1 1 1 ) axes in the plane of the photograph. 82 Preparation of ferrofluids A. 2 FeCl3 + FeCl2 + 8 NH3 + 4 H2O → Fe3O4 (s) + 8 NH4Cl (aq) B. Add cis-oleic acid [CH3(CH2)7CH=CH(CH2)7COOH ] in oil C. Remove water 83 A ferrofluid, influenced by a magnet underneath. 84 Electrorheological fluid – suspension of particles in a liquid medium whose viscosity can be tuned by an applied electric field 85 86 87 • An ER fluid consists of fine polarizable particles suspended in a fluid of lower dielectric constant. • Typically such fluids are assembled with a continuous hydrophobic liquid phase (e.g. silicone oil) containing hydrophilic particles (e.g. zeolite). • The density of the particles is matched as closely as possible with that of the oil to ensure good dispersion upon mixing of the ER fluid. • An applied electric field aligns the dipoles of water molecules trapped in particles, thus polarizing the particles. Particle polarization changes their organization in the fluid and causes changes in fluid rheological properties. 88 silicone oil -dimethylpolysiloxane hydrolyzate zeolite - crystalline aluminosilicate with open structures Type A 89 Nano-particles in confined space 90 91 X-Ray Powder Diffraction line-broadening technique Scherrer’s equation kλ d= β cosθ mean diameter of the crystallites diffraction angle line-broadening k: a constant, ~ 1 Dependent on shape, reflection indices, and definition of d, β λ: the radiation wavelength for CuKα, λ=1.5487 Å 92 Correction for instrumental broadening β 2 = B2 − b2 standard with particle d > 1,000 Å with diffraction line near the target line the breadth of the diffraction line If β define as β1/2, half-maximum line breadth K ~ 0.9 0.9λ d= β 1 cos θ 2 If correcting for strain and instrumental broadening, the min. particle diameter ~ 20 Å. Otherwise, ~ 50 Å 93 TEM technique 94 Physical processes for preparing nano-particles 95 96 97 98 99 Differential Scanning Calorimetry (DSC) φr (b) φr φr (T) T 熱流型圓盤狀 DSC 的(a)裝置:S= 樣品,R= 參比物,(1)金屬圓盤,(2)加熱爐,(3) 蓋子,(4)溫度感應器,(5)程溫控制器;(b)量測曲線,φr = - K·∆T。 100 功率補償型 DSC 的(a)裝置:S= 樣品,R= 參比物,(1)加熱器,(2)溫度感應器;(b) 控制電流環路,φm = - K·∆Tcalibrated。 101 DSC 曲線呈現的三種理想化的熱變化 102 103 Nano-material fabrication Lab Chip, 2005, 5, 492–500 104 105 Top-down Fig. 1 (a) Bulk-/film-machining; (b) hybrid-method for patterning. 106 107 108 Bottom-up Fig. 5 (a) Simplified schematics of the self-assembly peptide nanotubes (cylinder) in the membrane. (b) schematic representation of nanotube–vesicle networks. 109 phospholipid bilayer Cell Membrane- Proteins inserted into a phospholipid matrix 110 Molecular Assembly and SelfAssembly: Molecular Nanoscience for Future Technologies FIGURE 1. Chemical structure of (A) the Buckminster carbon-fullerene C60, (B) of Cu-tetra-[3,5 di-t-butylphenyl]-porphyrin (Cu-TBPP), and (C) of chloro-[subphthalocyaninato]boron(III) (SubPc). The molecular diameters are 1 nm, 1.3 nm, and 1.5 nm, respectively, and all three molecules have large delocalized -systems that are responsible for the Van der Waals attraction between the adsorbates and the substrates. Ann. N.Y. Acad. Sci. 1006: 291–305 (2003). 111 FIGURE 5. Self-intermixed monolayer phase, C60 and SubPc (see Fig. 1 A and C) codeposited on Ag(111), intermix and form a strongly anisotropic pattern. The C60s are adsorbed in a quasilinear arrangement, and the SubPcs act as spacers between these C60 lines. Image size, 9.7 x 9.7 nm2; sample bias, U = 1.9 V; tunneling current, I = 20 pA. On the right, the proposed model of the registry on Ag(111) is shown. The positions of the C60 and SubPc molecules and the orientations of the latter are depicted inside the unit cell. 112