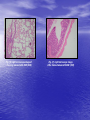
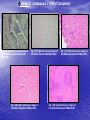
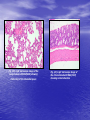
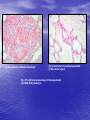
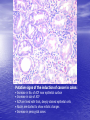
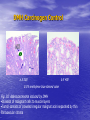
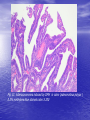
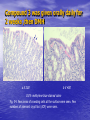
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Curcumin Analogs as Anticancer Agents
Discovery and development of TRPV1 antagonists wikipedia , lookup
CCR5 receptor antagonist wikipedia , lookup
Cannabinoid receptor antagonist wikipedia , lookup
Development of analogs of thalidomide wikipedia , lookup
Discovery and development of antiandrogens wikipedia , lookup
Drug discovery wikipedia , lookup
Theralizumab wikipedia , lookup
Discovery and development of tubulin inhibitors wikipedia , lookup
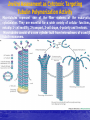
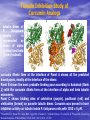
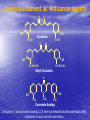
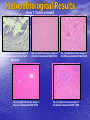
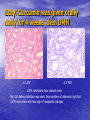
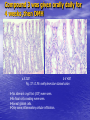
Targeted Drug Delivery System (TDDS): Encapsulating Newly Synthesized Anti-cancer Compounds-conjugated gold nanoparticles •Professor Dr. khairia Mohammed Youssef, Dept. of Pharm. Chemistry, Faculty of Pharmaceutical Sciences and Pharmaceutical Industries, Future University,P.O. BOX 12311, Cairo, Egypt •[email protected] •[email protected] • 1- Introduction • 2- Synthetic Methods of Curcumin and Curcumin analogs. • 3-In vitro Assessment of Curcumin and Curcumin • • • Analogs as Anti-Oxidant and Anti-Cancer Agents. 4-Mechanisms of Action of Curcumin and Curcumin analogs as Anti-oxidant and AntiCancer Agents. 5- Mechanism of Cytotoxic Tubulin Polimerization Activities. 6- In vivo Assessment of Curcumin as AntiCancer. – Preclinial Safety Evaluation in Mice and Rats. – Chemoprotective and Chemopreventive effects in DMH-Induced Colon Cancer in Albino Rats Model. 7- Conjugation with gold nanoparticles Introduction: Cancer and Curcumin as anticancer • Cancer is perhaps the most progressive and • • devastating disease posing a threat of mortality to the entire world despite significant advances in medical technology for its diagnosis and treatment. Recently, considerable attention has been focused on identifying naturally occurring chemopreventives. Wide arrays of phenolic substances, particularly those present in dietary and medicinal plants, have been reported to possess substantial anticarcinogenic and antimutagenic effects Curcumin diferuloylmethane, 1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione the major constituent of turmeric powder extracted from the rhizome of the plant curcuma longa Biological Properties of Curcumin and its Derivatives • • • • • • • • • 1. 2. 3. 4. 5. 6. 7. 8. 9. Antioxidant Anti-inflammatory Antibacterial, Antifungal and Antiparasitic Antiviral Antihistaminic Treatment of Skin Diseases Chemopreventive Effects Anticancer Treatment of Alzeheimer Synthetic Methods for the Preparation of Curcumin and Curcumin derivatives O RO CHO 1 NaOH/EtOH, NaOEt/EtOH 2 RO O + O or c.HCl OR 1 XXX HO O HO 1 OH In an attempt to prepare curcumin and curcumin analogs, search of the literature demonstrated that chalcones, Claisen-Schmidt condensation in general, are known to be prepared via the classic enolate condensation reaction which is an acyl addition reaction of a nucleophilic enolate to an electrophilic carbonyl carbon In 1973, Roughley and Whiting reported the instability of curcumin in alkaline medium and explained its alkaline degradation as shown in the following equations: alkaline degradation of Curcumin O O OCH 3 H3CO i OH HO O H3CO COOH H3CO + HO Feruloylmethane HO Ferulic acid i iv H3CO CHO CO2 (BaCO3) + HO vanillin acetone ii iii H3CO CH3COCH3 COOH CHI3 iodoform HO Vanillic acid iv CO2 (BaCO3) Roughley, P.J. and Whiting, D.A.; J. Chem. Soc. Trans I (1973), 2379-2388. Pabon Procedure for the Synthesis of Curcumin and its Analogs R1O 2 CHO O O (BuO)3B/ n-BuNH2 / B2O3 + HO O O R1O OR1 HO OH the acetylacetone-boric anhydride complex method was adopted successfully for the preparation of Hundreds of Curcumin and Curcumin Derivatives. • • • • Pabon, H.J.J; Rec. Tr. Chim. Pays-Bas (1964), 83: 379-386. Synthesis of Curcumin Analogues as Potential Antioxidant, Cancer Chemopreventive Agents. Khairia M. Youssef, Magda A. El-Sherbeny, Faiza S. El-Shafie, Hassan A. Farag, Omar A. Al-Deeb, Sit Albanat A. Awadalla. Arch. Pharm. Pharm. Med. Chem. 2004, 337, 42-54. Synthesis and Antitumor Activity of Some Curcumin Analogs. Part II. Khairia M. Youssef, Magda A. ElSherbeny; Arch. Pharm. Chem. Life Sci., 338, 181-189, 2005. Synthesis of bioconjugate analogs of curcumin as potent antitumor agents. Khairia M. Youssef, Omaima A. Abu-Alwafa, Reem I. Al-Wably. Med Chem Res (2012) 21:874–890. CH 3 H3C 2 + O O H3C B2O3 CH 3 O + O BO2- B O O (A) H3C CH 3 OCH 3 4 O HO n-BuNH2 H Cl- H+O HC=HC H3CO O O OCH 3 HO OH CH=CH B OCH 3 O (B) O HC=HC OCH 3 O+H CH=CH Cl- HCl O 2 H3CO O OCH 3 (C) HO OH + H2O Synthetic Methods for the Preparation of Curcumin Analogs O HO O OH OCH 3 OCH 3 • The Biological Study of Curcumin reported • the instability of curcumin at a pH above 6.5 which is attributed to the active methylene group. Omitting the active methylene group and one carbonyl group may lead to potent antioxidative compounds Synthesis of Curcumin Analogs O O CHO + R'O N OCH3 R N R'O OR'' R OR' OR'' R = H, Me, Et, Propyl, acetyl R' = H, Me, Et R''= H, Me, Et, alkyl derv. 3,5-Bis(substituted benzylidene)-N-alkyl-4-piperidones was obtained through condensation of the appropriate 1-alkyl-4-piperidone with the appropriate aldehyde under acidic condition. Prepared Compounds O O HO O HO OCH 3 N OCH3 CH3 OH O OCH 3 OH HO OCH3 N OC2H5 CH3 OH OC2H5 O O HO N OC2H5 • • • C2H5 OH OC2H5 HO N OCH3 CH3 OH OCH3 Synthesis of Curcumin Analogues as Potential Antioxidant, Cancer Chemopreventive Agents. Khairia M. Youssef, Magda A. El-Sherbeny, Faiza S. El-Shafie, Hassan A. Farag, Omar A. Al-Deeb, Sit Albanat A. Awadalla. Arch. Pharm. Pharm. Med. Chem. 2004, 337, 42-54. Synthesis and Antitumor Activity of Some Curcumin Analogs. Part II. Khairia M. Youssef, Magda A. ElSherbeny; Arch. Pharm. Chem. Life Sci., 338, 181-189, 2005. Synthesis and in vitro antioxidant activity of some new fused pyridine analogs. Mohamed A. Al-Omar, Khairia M. Youssef, Magda A. El-Sherbeny, Sit Albanat A. Awadalla, Hussein, I. El-Subbagh; Arch. Pharm. Pharm. Chem. Life Sci. 2005, 338, 181-189. Curcumin as Anti-Oxidant Agents O O H3CO OH OCH3 HO O H3CO OH OCH3 HO (a) OH (b) (1) Curcumin and curcumin derivative have a unique conjugated structures which show a typical radical trapping ability as a chainbreaking antioxidant Mechanism of Antioxidant Activity of Curcumin O O H3CO . O OCH3 O . AH: is the phenolic antioxidant. A●: is the antioxidant radical. X●: is another radical. species or the same as A●. During Metabolism, Biomolecules produce reactive peroxyl radicals during their oxidation, which may act as X● and couple with the antioxidant radical A● in the second step of the antioxidation process 1- In vitro Chromatographic Determination of Free Radical Scavenging Activity of Curcumin Analogs a: Diphenylpicrylhydrazyl Free Radical Test • Diphenylpicrylhydrazyl absorbs strongly at 515 nm. The reaction is carried out under pseudo-first-order conditions using excess of the tested compound compared to the reagent and monitoring the mixture for the decrease of absorption at 515 nm. • Form the linear regression line of the absorbency against time, the pseudo-first-order rate constant (Kapp) and the corresponding half-life time (t1/2) of the reaction was calculated. b- Chemiluminescence Measurement Scavenging Activity of Curcumin Analogs of Free Radical oxygen Produced by Peripheral multinuclear Neutrophil Cells (PMNs) • All of the synthesized compounds were tested for their ability to scavenge oxygen free radical produced by peripheral multinuclear neutrophil cells (PMNs) collected from apparently healthy blood donors. • Phorpol-12-myristate-13-acetate (PMA), in a final concentration of 2ng/ml was added to PMNs (5x105cells/ml) to stimulate respiratory burst which was magnified by luminol (10-4M) to be able to be measured by the LKB luminometer. • 100µg of synthesized drugs were added to detect its effect on the amount of oxygen radical liberated and the percentage of inhibition was calculated. Conclusion • The Anti-oxidant screening of most of Curcumin Analogs reveals that the results go hand by hand with the in vitro Anti-cancer effects results which performed at The National Cancer Institue (NCI). • The anti-oxidant and anti-cancer effects of these compounds depend mainly on the stabilization of the formed phenoxy free radical. The phydroxy phenyl moiety is very essential to produce the phenoxy free radical which is responsible for free radical scavenging effect. • o-Substitution by electron–donating group like o-methoxy group, increases stability of phenoxy free radical and hence increasing both free scavenging and antitumor effects. • o-Substitution by ethoxy group rather than methoxy, increases stability of phenoxy free radical and thus increasing both free scavenging and antitumor effects. • Increasing the alkyl group chain from methyl to ethyl on the N in the series of substituted N-alkyl piperidones, increases the activity toward both free radical scavenging and antitumor effects which may be attributed to increased positive inductive effect and / or increased lipophilicity of the new compounds. • Extension of conjugation, increases stabilization of phenoxy free radical. Chemopreventive Properties of Curcumin and Curcumin Analogs Mechanism of Action • Stimulation of phase I and phase II detox • • • • • • systems Inhibition of COX-1 and COX-2 enzymes Stimulation of glutathione-S-transferase Interference with cell growth by inhibition of protein kinases Neutralization of carcinogenic free radicals Curcumin significantly inhibits the activity of the isoenzymes of cytochrome P-450 involved in the metabolism of some carcinogens It inhibits SK-Hep-1 hepatocellular carcinoma cell invasion in vitro and suppresses matrix metalloproteinase-9 secretion • Cytotoxic Tubulin Polymerization Activities Invitro Assessment as Cytotoxic Targeting Tubulin Polymerization Activity Microtubules represent one of the fiber systems of the eukaryotic cytoskeleton. They are essential for a wide variety of cellular functions, notably: 1- cell motility, 2-transport, 3-cell shape, 4-polarity and 5-mitosis. Microtubules consist of a core cylinder built from heterodimers of α and β tubulin monomers. *Mary Ann Jordan & Leslie Wilson, “Microtubules as a target for anticancer drugs”. Nat. Rev. Cancer, (2004), 4: 253-265. Microtubules binding sites There are three different target sites on the tubulin heterodimer:the vinca alkaloid, the colchicine, and the paclitaxel binding sites. a. Vinblastine bound to highaffinity sites at the microtubule plus end suffice to suppress microtubule dynamics. b. Colchicine forms complexes with tubulin dimers and copolymerizes into the microtubule lattice, suppressing microtubule dynamics. c. A microtubule cut away to show the interior surface is shown. Paclitaxel binds along the interior surface of the microtubule, suppressing its dynamics. Tubulin Inhibition Study of Curcumin Analogs tubulin dimer of P. falciparum tubulin is represented as dimer of alpha (yellow) and beta (green) subunit. curcumin diketo form at the interface of Panel A shows all the predicted bound poses, mostly at the interface of the dimer. Panel B shows the most probable binding pose according to Autodock (Rank 1) with the curcumin diketo form at the interface of alpha and beta tubulin monomers. Panel C shows binding sites of colchicine (purple), paclitaxel (red) and vinblastine (brown) on parasite tubulin dimer. Curcumin was proved to have inhibition activity on tubulin inside P. falciparum cells with IC50 = 5 µM. *Chakrabarti R, Rawat PS, Cooke BM, Coppel RL, Patankar S, “Cellular Effects of Curcumin on Plasmodium falciparum Include Disruption of Microtubules”. PLoS ONE, (2013), 8 (3): e57302. SAR Performed on Novel Curcumin Analogs Docking Study Using Discovery Studio 2.5 and MOE Programs 1, 5-diaryl-3-oxo-1,4pentadienyl pharmacophore [Reacts at the primary binding site A of colchicine] O Auxiliary binding site B R' N R' O Groups capable of Van der Waal bonding Atoms capable of hydrogen bonding The group on N atom could lead to increases in cytotoxic potencies due to either additional binding with cellular constituents at an auxiliary binding site (site B) or by facilitating the interaction of the cytotoxin at site A. Newly Designed and Synthesized Curcumin Analogs with in vitro Cytotoxicity and Tubulin Polymerization Activity. Iten M. Fawzy, Khairia M. Youssef, Nasser S. M. Ismail, Joachim Gullbo and Khaled A. M. Abouzid, Chemical Biology & Drug Design. Early View, Article first published online: 25 NOV 2014. European Patent, PCT/EG 2013/000028. Invivo Assessment as Anticancer Agents O HO O Curcumin OCH 3 O O OH OCH 3 HO OH OCH 2CH 3 OCH 2CH 3 Ethyl Curcumin O HO CH 3 N CH 3 OH CH 3 Curcumin Analog Curcumin (1) and curcumin analogs (2,3) were synthesized and the preclinical safety evaluation in mice and rats were done. Preclinical Safety Evaluation in Mice and Rats Acute Toxicity (LD50) of tested compounds procedure Pilot experiments were performed by oral administration of increasing doses of tested compounds into different groups of mice (each consisted of 5 mice). Doses which produce 0 % and 100 % mortality were determined. Doses which used in the assessing of chemopreventive, antitumor effects Compound 1 (curcumin) Dose 0.05mg/gm/orally/day 2 3 0.12mg/gm/orally/day 0.16mg/gm/ora lly/day Litchfield JT, Wilconsxon F. J. Pharmcol. Exp. Ther. 1949, 96, 99-113. Blood Pressure and Electrocardigrahic Recording Test Control Curcumin 2 3 B.P. Mean 109 108.4 109.9 103.9* ± SEM ± 0.573 ± 0.57 ± 0.575 ± 0.546 Three groups of virgin Sprague-Dawley female rats, each consisted of 10 rats: Group A administered the calculated dose of compounds 1, 2 and 3 daily for two weeks. Group B administered the calculated dose of compounds 1, 2 and 3 daily for four weeks. Results of Blood Pressure and Electrocardigrahic Recording • These tests show that there is significant reduction in systolic B.P. only with pretreatment with compound 3 for 14 days P.O. administration at P , 0.05 while there is no changes in E.C.G. or heart rate. Histopathological Study • Different organs sections of untreated and treated animals e.g. mammary gland, heart, kidney, liver, spleen and colon were stained with routine hematoxylin and eosin (HX and E) to be examined under light microscope (Fig. 2-7) Histopathological Results Group 1: Control (untreated) Fig. (1) Light microscope image of the Mammary gland stained with H&E (X20) Fig. (2) Light microscope image of the Heart stained with H&E (X10) Fig. (4) Light microscope image of the Liver stained with H&E (X20) Fig. (3) Light microscope image of the Kidney stained with H&E (X40) Fig. (5) Light microscope image of the Spleen stained with H&E (X20) Fig. (6) Light microscope image of the Lung stained with H&E (X20) Fig. (7) Light microscope image ofthe Colon stained with H&E (X20) Group 2 : Compound 1 (Curcumin) After Four Weeks Fig. (8) Light microscope image of the Mammary gland stained with H&E (X20) Fig. (9) Light microscope image of the Heart stained with H&E (X10) Fig. (11) Light microscope image of the Liver stained with H&E (X20) Fig. (10) Light microscope image of the Kidney stained with H&E (X40) Fig. (12) Light microscope image of the Spleen stained with H&E (X20) Fig. (13) Light microscope image of the Lung stained with H&E (X20) Fig. (14) Light microscope image of the Colon stained with H&E (X20) showing normal structure Group 3: Compound 2 (Ethyl Curcumin) Fig. (15) Light microscope image of the Mammary gland stained with H&E (X20) Fig. (16) Light microscope image of the Heart stained with H&E (X10) Fig. (18) Light microscope image of the Liver stained with H&E (X20) Fig. (17) Light microscope image of the Kidney stained with H&E (X40) Fig. (19) Light microscope image of the Spleen stained with H&E (X20) Fig. (20) Light microscope image of the Lung stained with H&E(X20) showing thickening of the interestial space Fig. (21) Light microscope image of the Colon stained with H&E (X10) showing normal structure Group 4: Compound 3 Fig. (22) Light microscope image of the Mammary gland stained with H&E (X20) Fig. (23) Light microscope image of the Heart stained with H&E (X10) Fig. (25) Light microscope image of the Liver stained with H&E (X20) Fig. (24) Light microscope image of the Kidney stained with H&E (X40) Fig. (26) Light microscope image of the Spleen stained with H&E (X20) (a) Accumulation of fluids in the alveoli (b) Accumulation of lymphocytes and RBC in the alveolar space Fig. (27) Light microscope image of the Lung stained with H&E (X20) showing in (a) X10 (b) X40 Fig. (28) Light microscope image of the Colon stained with H&E showing an increase in the goblet cells in the base of the crypts (arrows) Conclusion compound 1 (curcumin) and compound 2 (ethyl curcumin showed normal structure after 4 weeks treatment identical of that in 2 weeks . Compound 3 shows some intraalveolar lung haemorhage after four weeks. Chemoprotective Effect of Curcumin and its Analogs in DMH-Induced Colon Cancer in Albino Rats Model. Bird RP. Role of aberrant crypt foci in understanding the pathogenesis of colon cancer .Cancer Letters 1995; 93 : 55-71. Histological evaluation and Aberent Cript Focy assay • Group A: animals constituted the normal • • • • untreated controls Group B: Comprised of carcinogen control animals. Dimethylhydrazine(DMH) was administered subcutaneously, at a dose of 20mg/kg-body weight ,once a week in 0.9%Na Cl solution(pH 7.2) for a total period of 30 weeks . Chemoprotective treatment part(1) Two weeks then DMH was administered Chemoprotective treatment part(2) Four weeks then DMH was administered Normal untreated control Fig. 29: No signs of abnormality 0.3% methylene blue stained colon . X 200 Putative signs of the induction of cancer in colon: • • • • • Increase in No. of ACF near epithelial surface Increase in size of ACF ACF are lined with thick, deeply stained epithelial cells Nuclei are started to show mitotic changes Increase in pericryptal zones DMH Carcinogen Control a X 200 b X 400 0.3% methylene blue stained colon Fig. 30: Adenocarcenoma induced by DMH •Invasion of malignant cells to muscle layers •Tumor consists of crowded irregular malignat acini separated by thin fibrovascular stroma Fig. 31: Adenocarcenoma induced by DMH in colon (adenomatous polyps ) 0.3% methylene blue stained colon X 200 Curcumin was given orally daily for 2 weeks ,then DMH a X 200 b X 400 0.3% methylene blue stained colon Fig. 32: Crowding cells at the surface with enlarged and hyperchromatic nuclei with scattered aberrant crypt foci (ACF) were seen. Ethyl Curcumin was given orally daily for 2 weeks ,then DMH a X 200 b X 400 0.3% methylene blue stained colon Fig. 33: Moderate protection was seen. Crowding cells at the surface with enlarged and hyperchromatic nuclei with scattered aberrant crypt foci (ACF) were seen. Compound 3 was given orally daily for 2 weeks ,then DMH a X 200 b X 400 0.3% methylene blue stained colon Fig. 34: Few areas of crowding cells at the surface were seen. Few numbers of aberrant crypt foci (ACF) were seen. Curcumin was given orally daily for 4 weeks ,then DMH a X 200 b X 400 0.3% methylene blue stained colon Fig. 35: Few areas of crowding cells at the surface were seen. Few numbers of aberrant crypt foci (ACF) were seen. Ethyl Curcumin was given orally daily for 4 weeks ,then DMH a X 200 b X 400 0.3% methylene blue stained colon Fig. 36: Beter protection was seen. Few numbers of aberrant crypt foci (ACF) were seen with less sign of neoplastic changes. Compound 3 was given orally daily for 4 weeks ,then DMH a X 200 b X 400 Fig. 37: 0.3% methylene blue stained colon No aberrant crypt foci (ACF) were seen. No focal cell crowding were seen. Normal globlet cells. Only some inflammatory cellular infiltration. Aberrant Crypt Foci (ACF) Assay Chemoprotective efficay of different forms of curcumin and curcumin analogs administered for 2 weeks orally before DMH-induced Aberrant crypt foci(ACF) in male wistar rats . No of ACF/Rat colon Inhibition% (mean+S.E) Group No. of rats/G N.control (A) 10 ------- ----- DMHcontrol(B) 10 100.5+2.54 0 DMH+ curcumin 10 54.3 +1.26 45.98 DMH + ethyl curcumin 10 51.5 +1.20 48.5 DMH + C3 10 37.4 +0.95 62.6 N: Normal control received saline s.c. once weekly for 30 weeks. DMH : was given at a dose of 20mg/kg s.c once weekly for30 weeks. DMH+C1 :}Curcumin was given at a dose of 53mg/kg orally once daily orally. DMH +C2 : Ethyl curcumin was given at a dose of 123mg/kg orally once daily . DMH +C3 : compound 3 was given at a dose of 161 mg/kg orally once daily C1,C2, C3 were given 2 weeks before induction of cancer colon by DMH. Chemoprotective efficay of different forms of curcumin and curcumin analogs administered for 4 weeks orally before DMH-induced Aberrant crypt foci(ACF) in male wistar rats . Group No of rats/G No of ACF/ Inhibit Rat colon(mean+S.E) % N.control(A) 10 ----- ---- DMH control(B) 10 100.5+2.54 0 DMH+ curcumin 10 47.7+1.38 52.3 DMH+ ethyl curcumin 10 45.8 + 1.71 54.2 DMH+C3 10 ----- 100 N: Normal control received saline s.c once weekly for 30 weeks . DMH: was given at a dose of 20mg/kg s.c once weekly for 30 weeks. DMH+ C1: curcumin was given at a dose of 53mg/kg orally daily . DMH+C2 : ethyl curcumin was given at a dose of 123mg/kg orally daily. DMH+ C3 : compound 3 was given at a dose of 161mg/kg orally daily. C1,C2,C3 were given daily for 4 weeks before induction of cancer colon by DMH. Chemoprotective efficacy of different forms of curcumin and curcumin analogs administered for 2 weeks orally before DMH-induced colonic cancer in rats . No of rats /G No.of colon tumors 1 N.control(A) 10 ------- DMH control(B) 10 20 DMH+curcumin 10 8 DMH+ethyl curcumin 10 6 DMH+C3 10 2 N: normal control rats received saline s.c. once weekly for 30 weeks. DMH: was given at a dose of 20mg/kg s.c. once weekly for 30 weeks . DMH +C1: curcumin was given at a dose of 53mg/kg orally once daily for 2 weeks. DMH+C2: ethyl curcumin was given at a dose of 123mg/kg orally once daily for 2 weeks . DMH+C3 : compound 3 was given orally at a dose of 161mg/kg once daily Chemoprotective efficacy of different forms of curcumin and curcumin analogs administered for 4 weeks orally before DMH –induced colonic cancer in rats No. of rats /G No.of colon tumors/G N.control 10 --- DMH control(B) 10 20 DMH+curcumin 10 4 DMH +ethyl curcumin 10 2 DMH+C3 10 -- N: normal control rats received saline s.c. once weekly for 30 weeks . DMH : was given at a dose of 20mg/kg s.c. once weekly for 30 weeks. DMH+C1 : curcumin was given at a dose of 53 mg/kg . DMH+C2 : ethyl curcumin was given at a dose of 123 mg/kg . DMH+C3 : compound 3 was given at a dose of 161mg/kg . Chemopreventive Effects in DMH-Induced Colon Cancer in Albino Rats Model Histopathological Examination of Chemopreventive efficacy of curcumin and curcumin analogs administered for 2 weeks orally after DMH-induced colonic cancer in rats No. of rats /G No.of colon tumors/G DMH control(B) 10 20 DMH+C1 (C) 10 8 DMH+C2 (D) 10 6 DMH+C3 (E) 10 2 N: normal control rats received saline s.c. once weekly for 15 weeks. DMH: was given at a dose of 20mg/kg s.c. once weekly for 15 weeks . DMH +C1: compound 1 was given at a dose of 53mg/kg orally once daily for 2 weeks. DMH+C2: compound 2 was given at a dose of 123mg/kg orally once daily for 2 weeks .DMH+C3 : compound 3 was given orally at a dose of 161mg/kg once daily Chemopreventive efficacy of of curcumin and curcumin analogs administered for 4 weeks orally after DMH –induced colonic cancer in rats No. No.of ofrats rats/G /G No.of No.ofcolon colontumors/G tumors/G N.control N.control 10 10 10 ----- DMH DMHcontrol(B) control(B) 10 10 20 20 DMH+C1(F) DMH+C1(F) 10 10 44 DMH DMH+C2(G) +C2(G) 10 10 22 DMH+C3(H) DMH+C3(H) 10 10 --- N: normal control rats received saline s.c. once weekly for 15 weeks . DMH : was given at a dose of 20mg/kg s.c. once weekly for 15 weeks. DMH+C1: compound 1 was given at a dose of 53mg/kg. DMH+C2 :compound 2 was given at a dose of 123mg/kg . DMH+C3 : compound 3 was given at a dose of 161mg/kg . Conclusion • In conclusion, the results of this study suggest that daily supplementation of Curcumin and Curcumin Analogs has a positive beneficial effect against chemically induced colonic preneoplastic progression in rats induced by DMH, which provide an effective dietary chemoprotective and chemopreventive approach to disease management . • Best results were given with compound (3). Conjugation with gold nanoparticles • The result of the previous work inhanced us to use the Targeted drug delivery system (TDDS), especially gold-based nanoparticles (AuNPs) to be used as a model system in this work. • The 1.4 nm Nanogold® particle is a gold compound: it is not just adsorbed to proteins, like colloidal gold, but covalently reacts at specific sites under mild buffer conditions. • The main objective is that, the specific conjugated drug is targeted to fit and improve their binding affinity, to the specific receptor that ultimately will trigger the pharmacological activity as anticancer. • The other objective is the dual destructive effect on cancer cells after releasing of gold nanoparticles from the drug. • In this work, the highly stable mercapto capped gold nanoparticles were prepared adopting Scheme 1 Synthesis and Conjugation with Gold Nanoparticles 1- Synthesis of biocompatible gold nanoparticles (GNPs) with different sizes and shapes 2- Physiochemical characterization of the synthesized GNPs using TEM, XRD, DLS, …etc. techniques 3- Conjugation of the intended drugs with different types of GNPs (different sizes & shapes) 4- Physiochemical characterization of the synthesized Drug/GNPs nanocomposite using TEM, XRD, DLS, …etc. techniques to confirm the formation of the conjugate. 5- Studying the optimization condition such as effect of Temp., pH, loading concentrations…etc. to achieve the most probable (stable) formula of the Drug/GNPs nanocomposite. 6- Studying the rate of drugs release at variable conditions 7- Pharmaceutical formulation of the final product • The superior stability of these particles under the conditions of varied pH and electrolyte concentrations will be studied. • In addition to developing synthetic methodology that is free from reducing agents and elevated temperature, we will prob the interactions between the gold core and the stabilizer molecules using Fourier transformed infrared spectroscopy (FTIR) technique (will be done at University of Georgia Technology Labs). • Long term stability of newly synthesized anti-cancer compounds capped GNPs may provide improved shelf life. While the controlled release and sustained delivery of the anti-cancer drugs from the gold core is demonstrated with the help of GSH of varying concentrations. • In vitro and In vivo anti-cancer activity of released compounds against different types of cancer cells will be investigated in detail. Reasons behind enhanced anti-cancer activity will be also addressed in this work. Scheme 1 O RO H O OR O N R' H P2 S 5 CS2 R = CH3, CH5 R' = H, COCH3, CONH2 O RO H S OR N R' S H Au nanoparticles O RO nanoparticles Au S OR N R' S Au nanoparticle Conjugated compounds with gold nanoparticles Molecular docking studies of tubulin inhibitors • Docking Study was performed using the MOE software. • Downloading the crystal structure of CDK2 enzyme complexes with inhibitor was carried out from protein data bank website (PDB). • Regularization and optimization for protein and ligand were performed. • Determination of the essential amino acids in binding site was carried out and compared with that present in literature. • The performance of the docking method was evaluated by redocking crystal ligand into the assigned active site of to determine the root mean square deviation (RMSD) value. • Interactive docking was carried out for all the conformers of each compound of the test set to the selected active site. • Each docked compound was assigned a score according to its fit in the ligand binding pocket (LBP) and its binding mode. ligand podophyllotoxin E Score = -11 K cal/mol Comp. k1 E Score = -9.8 K cal/mol Comp. k2 E Score = -10.9 K cal/mol Comp. k3 E Score = -11.9 K cal/mol Comp. k4 E Score = -11 K cal/mol Comp. k5 E Score = -10.5 K cal/mol Comp. k6 E Score = -10 K cal/mol Conclusion Comp. k3 E Score = -11.9 K cal/mol Patents and Published Papers 1- U.S. Patent No. 60/670,844, filed April 13, 2005. 2- Egyptian Patent No. 1131/2008, filed July 3, 2008. 3- European Patent, PCT/EG 2013/000028. 1. Synthesis of Curcumin Analogues as Potential Antioxidant, Cancer Chemopreventive Agents. Khairia M. Youssef, Magda A. El-Sherbeny, Faiza S. El-Shafie, Hassan A. Farag, Omar A. Al-Deeb, Sit Albanat A. Awadalla. Arch. Pharm. Pharm. Med. Chem. 2004, 337, 42-54. 2. Synthesis and Antitumor Activity of Some Curcumin Analogs. Part II. Khairia M. Youssef, Magda A. ElSherbeny; Arch. Pharm. Chem. Life Sci., 338, 181-189, 2005 3. Synthesis and in vitro antioxidant activity of some new fused pyridine analogs. Mohamed A. Al-Omar, Khairia . Albanat A. Awadalla, Hussein, I. El-Subbagh; Arch. Pharm. Pharm. M. Youssef, Magda A. El-Sherbeny, Sit Chem. Life Sci. 2005, 338, 181-189. 4. Curcumin Analogs as Anticancer Agents: 1) Preclinical Safety Evaluation in Mice and Rats. 2) Chemopreventive Effects in DMH-Induced Colon Cancer in Albino Rats Model. Khairia M. Youssef, Azza M. Ezzo, Moushira I. El-Sayed, Amal A. Hazzaa, Azza H. EL-Medany, Maha Arafa. The International Symposium on Recent Progress in Curcumin Reseach, September 11-12, 2006 at Yogyakarta, Indonesia. 5. PAC, a novel curcumin analogue, has anti-breast cancer properties with higher efficiency on ER-negative cells Ensaf M. Al-Hujaily, Ameera Gaafar Mohamed, Ibtehaj Al-Sharif, Khairia M. Youssef and Pulicat S. Manogaran, et al. Breast Cancer Res Treat (2011) 128:97–107 6. Synthesis of curcumin and ethylcurcumin bioconjugatesas potential antitumor agents Reem I. Al-Wabli Omaima M. AboulWafa Khairia M. Youssef Med Chem Res (2012) 21:874–890 7. Molecular Modeling of Novel Curcumin Analogs with Anticipated Anticancer Activity: FUE International Conference on Pharmaceutical Technologies (ICPT) 2012.Iten M. Fawzy, Khairia M. Youssef, Nasser S. M. Ismail, Khaled A. M. Abouzid. 8. Design and Synthesis and Biological evaluation of Novel Curcumin Analogs with anticipated anticancer activity. Iten M. Fawzy, Khairia M. Youssef, Nasser S. M. Ismail, J. Gullbo and Khaled A. M. Abouzid. Journal of Future University in Egypt 2014. In Press. 9. Molecular docking and in silico ADME study of Novel N9-substituted Purines targeting CK1 and abl-tyrosine kinase. Iten M. Fawzy, Khairia M. Youssef, Nasser S. M. Ismail , Deena S. Lasheen and Khaled A. M. Abouzid2. FIP conference 2014 at Bangkok. 10. Newly Designed and Synthesized Curcumin Analogs with in vitro Cytotoxicity and Tubulin Polymerization Activity. Iten M. Fawzy, Khairia M. Youssef, Nasser S. M. Ismail, Joachim Gullbo and Khaled A. M. Abouzid, Chemical Biology & Drug Design. Early View, Article first published online: 25 NOV 2014. Acknowledgement • Taher Salah, Professor of Nanotechnology, Manager of • • • • • • Nanotechnology & Advanced Materials Central Lab. (NAMCL).Regional Center for Food & Feed (RCFF), Agricultural Research Center (ARC). Nasser Saad, Asst. Prof. Department of Pharmaceutical Chemistry, Future University in Egypt. Azza Ahmed, Asst. Prof. Department of Pharmaceutical Technology, Future University in Egypt. Azza M. Ezzo, Pharmacology Dept., Faculty of Medicine, AlAzhar University, Cairo, Egypt. Maha Arafa, Histopathology Dept., College of Medicine, King Saud University, Riyadh, Saudi Arabia. King Saud University, Saudi Arabia. Future University In Cairo.