* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Common Cardiac Surgeries Children
Mitral insufficiency wikipedia , lookup
Aortic stenosis wikipedia , lookup
History of invasive and interventional cardiology wikipedia , lookup
Myocardial infarction wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Cardiac surgery wikipedia , lookup
Coronary artery disease wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Atrial septal defect wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
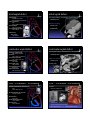
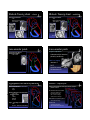
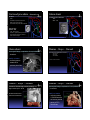
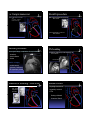
Common Cardiac Surgeries Disclosure in Children Dianna M. E. Bardo, M. D. Consultant & Speakers Bureau – honoraria Director of Cardiac Radiology Associate Professor of Radiology, Pediatrics, & Cardiovascular Medicine Koninklijke Philips Healthcare definitions & a reality check normal cardiac circulation Malformation the right and left sides are normally separate circulations A primary structural defect arising from a localized error in morphogenesis - results in the abnormal formation of an organ separated by the pulmonary capillary bed intracardiac & extracardiac t di shunts h t Dysplasia Refers to an abnormal organization g of cells into tissues - results in abnormal tissues The distinction of malformation from dysplasia is at best blurry – there is much overlap repair complete anatomic correction of congenital heart defect palliation provides physiologic correction of blood flow in utero – 2 normal shunts foramen ovale ductus arteriosus postnatal abnormal shunts ASD VSD truncus arteriosus PDA patent ductus arteriosus patent ductus arteriosus division & over-sewing Performed when he was Chief Resident & his surgical chairman was out of town! 1938 Gross – Children’s Hospital Boston post-surgical findings, complications & re-op indications essentially none need for re-imaging virtually none triple ligation technique 1946 Blalock – Johns Hopkins pharmacologic closure indomethacin 1976 Heymann Catheter based coil or device closure 1993 atrial septal defect atrial septal defect patent closed technique foramen ovale post-surgical findings, complications & re-op indications essentially none lateas1940s earlypressures 1950s closes right &heart increase postnatally Bailey & Sondergaard (separately) open repair technique primum1952 – AVSD Gross – Children Children’ss Hospital Boston direct visualization 1953 – Lewis & Taufic secundum using cardiopulmonary bypass 1954 – Gibbons need for re-imaging virtually none residual ASD sinus venosus Catheter based device closure 1997 – Matsura ventricular septal defect ventricular septal defect PAbanding – palliation of VSD membranous 1952 – Muller & Dammann paramembranous VSD closure 1954 – Lillehei – U of Minnesota muscular using a heart-lung machine 1955 – Kirklin – Mayo Clinic singleclosure transatrial 1958 –Stirling multiple total circulatory arrest 1969 – Okamoto spontaneous deep hypothermia & closure arrest Barratt-Boyes Amplatz closure device surgical & instrumented closure 1999 - Thanopoulos post-surgical findings, complications & re-op indications essentially none TOF – PA atresia – PA stenosis TOF – PA atresia – PA stenosis Prosthetic conduit between subclavian & PA 1962 Kilner – refined by Leval need for re-imaging virtually none residual VSD in utero blood flow is supplied to the lungs via the ductus arteriosus BT shunt ipsilateral to the aortic arch Laks and Castaneda DAo to PA shunt 1946 – Potts Central aortopulmonary shunt 1955 – Davidson AAo to PA shunt 1962 – Waterston post natal pulmonary vascular resistance is high requires arterial pressure to perfuse the lungs ductus arteriosus closes . . . or . . . maintained opened with PGE Blalock-Taussig shunt – classic Blalock-Taussig shunt – modified commonly used developed for ‘bluetemporary babies’ shunt commonly used developed for ‘bluetemporary babies’ shunt 1945to palliate low pulmonary blood flow ( TOF, PA atresia) designed Taussig Thomas) directsBlalock arterial&blood flow(&from a subclavian artery to pulmonary arteries Johns Hopkins used to augment PA blood flow while PA pressures transition from elevated perinatal pressure to normal TOF tricuspid atresia DORV other single ventricle physiology 1945to palliate low pulmonary blood flow designed Taussig Thomas) directsBlalock arterial&blood flow(&from a subclavian artery to pulmonary arteries Johns Hopkins used to augment PA blood flow while PA pressures transition from elevated perinatal pressure to normal TOF tricuspid atresia DORV other single ventricle physiology trans-annular patch trans-annular patch xaugmentation of the RVOT & surgical complications inadequate relief of obstruction pulmonary insufficiency enlargement of the MPA 1986 – Kirklin need for re-imaging restenosis of RVOT branch PA stenosis RV failure due to PI Hypoplastic left heart syndrome HLHS – staged repair all sided structures are small RPAleft – AAo anastomosis 1970 mitral valve Cayler left ventricle Multiple of this anastomosis aorticmodifications valve 1977 – 1981 ascending aorta Doty staged surgical procedures toward goal of Fontan circulation palliation of HLHS Levitsky Behrendt coronary artery perfusion is Norwood via retrograde flow from the Stage 1 – proceeding to successful Fontan ductus arteriosus through 1983 the ascending Norwood – aorta Children’s Hospital Boston neo-aorta & BT shunt are created anastomosis of MPA to AAo limit pulmonary blood flow ASD – created or enlarged arterial pressure to the lungs bidirectional cavo-pulmonary shunt venous pressure to lungs Fontan circuit completed circuit delivers SVC & IVC blood flow to the lungs Norwood procedure – alternatives Glenn shunt Sano shunt 2003 permanent shunt circulatory bypass of the R heart Distal MPA is separated from the heart MPA is used to create neo-aorta shunt between the systemic RV and the PAs Hybrid procedure Akintuerk – 2002 2004 – Bacha & Hijazi PA bands – regulate pulmonary blood flow Stent maintains patent ductus arteriosus ASD is made or enlarged Glenn shunt post-surgical findings, complications & re-op indications • thrombosis need for re-imaging • confirming patency • assessment of 1958to palliate hypoplasia of intended Glenn – Yale R sided structures unilateral il t l bilateral bidirectional used to augment PA blood flow after PA pressures have normalized Damus – Kaye – Stansel Anastomosis of AAo & MPA & RV to PA conduit 1975 – Damus 1975 – Kaye 1975 – Stansel Variation on the Norwood Stage 1 anastomosis of the hypoplastic ascending aorta to the native MPA pulmonary blood flow Damus – Kaye – Stansel Damus – Kaye – Stansel correction of TGA with single ventricle physiology – or single ventricle repair – HLHS post-surgical findings, complications & re-op indications • thrombosis the MPA is transected and anastomosed with the AAo need for re-imaging • confirming patency of DKS anastomosis and coronary arteries • patency of BT & Glenn shunts Fontan circulation Fontan circulation Multi-staged procedure to palliate tricuspid atresia, single Superior and inferior vena cavae anastomosis to the PAs [HLHS, HRV with PA atresia] ventricle syndromes 1971 total cavopulmonary connection post-surgical findings, complications & re-op indications • thrombosis • pleural effusions • ascites Fontan – University of Bordeaux returns systemic venous blood flow to the lungs separate from right heart contractions The R & L circulations are separate Transposition of the great arteries D – TGA AV concordance VA discordance p parallel circulations requires mixing - shunt L – TGA AV discordance VA discordance need for re-imaging • confirming patency • assessment of pulmonary blood flow Jatene arterial switch Arterial switch operation 1975 – Jatene of Correction D loop TGA at the arterial level physiological correction of TGA AP window & baffling to MPA are switches the aorta and the coronary arteries and the coronary arteries are 1978 – Aubert reimplanted i l d iinto the h neo aorta Translocation of aortic root including coronary origins 1980 – Bex 2 wrongs do not make a right Jatene arterial switch Jatene arterial switch correction of D loop TGA at the arterial level post-surgical findings, complications & re-op indications • tension on great vessels & reimplanted coronary arteries physiological correction of D-TGA the aorta and MPA are switched and the coronary arteries are reimplanted into the neo aorta need for re-imaging • coronary artery origin stenosis • RV failure as it is not well suited to be the systemic ventricle Le Compte maneuver Rastelli procedure transfer of PAs to the AAo maneuver toanterior minimize kinking 1981 – Institute can beLea Compte complication ofofthe Research & Surgery MPA is ligatedof and anastomosed to the RV correction TGA with VSD 1969 – Rastelli and LVOT obstruction of the coronary arteries which arterial switch Jatene procedure pulmonary arteries are ddrapedd over th the AA AAo RV – PA conduit is also used for PA atresia, atresia TOF, TOF DORV, or HLHS bovine pericardial conduit or tunnel connecting the LV to from the aorta artificial graft material – Borromee the RV1988 to the PAs Rastelli procedure post-surgical findings, complications & re-op indications • thrombosis • pleural effusions • ascites PA banding circumference D or Lof transposition band (mm) = child’s weight (kg) + 20 Trusler & Mustardaortic outflow – AV or subaortic stenosis obstructed decreases PA blood flow protects the pulmonary vascular bed need for re-imaging • conduit stenosis • pulmonary insufficiency • RV hypertrophy & failure Mustard or Senning – atrial switch correction of D-TGA at the atrial interatrial baffle 1954 – Mustard using artificial intra-atrial baffle pericardium directs pulmonary venous return level double switch Physiologic correction of congenitally corrected L-TGA to the systemic ventricle & systemic venous return to the right ventricle disadvantage – leaves the–RV to supply the 1959 Senning systemic using circulation atrial tissue Senning or Mustard & Jatene or Rastelli Ross procedure Coarctation of the aorta Used to valve treatmoved to the Pulmonic aortic position aortic stenosis 1962 – Ross Guys Hospital – London first surgical repair of coarctation of the aorta 1944 Crafoord – Karolinska Institute may include replacement of a portion of the AAo coronary arteries are transferred cadaveric homograft is used to replace the native pulmonic valve Coarctation of the aorta – surgical Coarctation of the aorta – stent End – to – end anastomosis Interventional – catheter based repair most often performed during the first year of life tissues are more elastic so bringing ends together easier may be an oblique anastomosis Patch repair performed at any age Aorto-aorto bypass graft used to palliate interrupted aortic arch OR tto supplement l t repaired i d coarctation of the aorta angioplasty & stenting to dilate coarctation of the aorta beware of jailing of the left subclavian artery origin Waterston & Potts shunts not currently performed augments pulmonary arterial blood flow sometimes excessively so Waterston shunt AAo – RPA Pott’s shunt DAo - LPA Waldhausen procedure Repair of aortic coarctation Left subclavian artery is ligated and used to augment the stenotic aorta Complications reduced blood flow to the left upper extremity poor growth of the extremity no longer used Common Surgical Procedures for Congenital Heart Disease Dianna M. E. Bardo, M. D. Director of Cardiac Radiology Associate Professor of Radiology, Pediatrics, & Cardiovascular Medicine