* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Topic0990 Electrical Units In attempting to understand the properties
Potential energy wikipedia , lookup
Maxwell's equations wikipedia , lookup
Work (physics) wikipedia , lookup
Fundamental interaction wikipedia , lookup
History of electromagnetic theory wikipedia , lookup
Electrical resistance and conductance wikipedia , lookup
Electrical resistivity and conductivity wikipedia , lookup
Lorentz force wikipedia , lookup
Circular dichroism wikipedia , lookup
Electromagnetism wikipedia , lookup
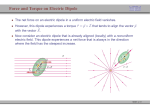
Topic0990 Electrical Units In attempting to understand the properties of chemical substances, chemists divide chemistry into two parts. In one part, chemists are interested in understanding intramolecular forces which hold molecules together. For example, using quantum mechanics and associated theories of covalent bonding, chemists describe the cohesive forces holding carbon, hydrogen and nitrogen atoms together in cyanomethane, CH3CN. At ambient temperature and pressure, cyanomethane is a liquid. In the second part of the sub-division of chemistry, chemists describe the intermolecular forces [1] which hold assemblies of molecules together in, for example, liquid and solid states; e.g. those forces which hold CH3CN molecules together in the liquid state. Common experience tells us that intermolecular forces are weaker than intramolecular forces. When we heat CH3CN( l ) at ambient pressure, the liquid boils at a characteristic temperature to form a vapour. The intermolecular separation dramatically increases but the covalent bonds within CH3CN do not break. [Of course, these bonds break at very high temperatures - thermolysis.] Here the emphasis centres on intermolecular cohesion. But this cannot be the whole story. If cohesion is the only force operating, molecules would collapse into each other in some nuclear catastrophe. This does not happen. Opposing the forces of cohesion are repulsive forces. In fact everyday experience leads to the idea of "size"; 'size is repulsive'. Basic Physics Molecules contain charged particles; protons (with positive electric charge) and electrons (with negative electric charge-- by convention). Intermolecular forces are understandable in terms of equations describing electrical interactions between electrically charged particles. An SI base unit is the ampere; symbol = A [2]. The SI unit of electric charge is the coulomb (symbol = C) defined as A s [3]. Electric Current An electric current I is driven through an electrical resistance R, by an electric potential gradient across the resistance. An ammeter measures the electric current I. The voltmeter records the electric potential gradient, ∆E across the resistance. The property called resistance R is given by Ohm’s Law; ∆E = I ⋅ R . (a) In the IUPAC system the unit of resistance is ohm [symbol Ω ≡ V A −1 ]. The electric potential difference is measured in volts, symbol V [4]. Electrical Capacitance In a simple electric circuit, a small battery is connected across a parallel plate capacitance. No current flows in this circuit. The battery produces a set of equal in magnitude but opposite in sign electric charges on the two plates. A capacitance stores electric charge. In practice the extent to which a capacitance stores charge depends on the chemical substance between the two plates. This substance is characterised by its electric permittivity; symbol = ε . Where a vacuum exists between the two plates, the electric permittivity equals ε0 [5]. The permittivity of a liquid is measured by comparing capacitance C when the gap between the plates is filled with this liquid and with capacitance C0 when the gap is "in vacuo". Then or ε r = ε / ε 0 = C / C0 (b) C = ε r ⋅ Co (c) For all substances, εr is greater than unity. In other words, with increase in εr so the electrical insulating properties of the system increase. At this stage, we have not offered a molecular explanation of the properly called εr but we have indicated that εr be can measured [6,7]. Intermolecular Forces and Energies Molecule i and molecule j are separated by a distance r; we assert that r >> molecular radii of molecules i and j. Our discussion centres on the assertion that a force (symbol X) exists between the two molecules. Moreover, this force depends on the distance of separation r. Thus X = f(r) (d) Ion-Interactions The force X between two electric charges q1 and q2 distance r apart ‘in vacuo’ is given by equation (e); (Couloumb's Law) [8]. X = q1 ⋅ q 2 / 4 ⋅ π ⋅ ε 0 ⋅ r 2 (e) Two ions, i and j, have charge numbers zi and zj respectively [for K +, zj = + 1; for SO42- , zj = -2]. For two ions ‘in vacuo’, the interionic force is given by equation (f). Fij = ( z i e ) ⋅ ( z j e) / 4π ⋅ ε 0 ⋅ r 2 (f) r But pairwise potential energy, U ij = − ∫ Fij ⋅ dr (g) r =∞ Hence, U ij = ( z i e ) ⋅ ( z j e ) / 4π ⋅ ε 0 ⋅ r (h) Equation (h) yields the interaction potential energy between a pair of ions [9]. The result is an energy expressed in joules. However, there are often advantages in considering an Avogadro number (i.e. a mole) of such pairwise interactions. U ij / J mol −1 = N A ⋅ ( z i e ) ⋅ ( z j e ) / 4π ⋅ ε 0 ⋅ r (i) We consider two classes of ion-ion interactions: (i) Ions i and j have the same sign For cation-cation and anion-anion pairwise interactions the force between the ions is repulsive. The pairwise potential energy increases with decrease in ion-ion separation. To bring two ions having the same charge closer together we have to do work on the system, increasing the pairwise potential energy Uij. (ii) Ions i and j have opposite signs For this system, ( z i ⋅ z j ) < 0 . Ion-ion interaction is attractive and the potential energy Uij decreases with decrease in rij. We write z i ⋅ z j to indicate the modulus of the product of the charge numbers. U ij / J mol −1 = − N A ⋅ z i ⋅ z j e 2 / 4π ⋅ ε 0 ⋅ r (j) Hence Uij has a (1/r) dependence on distance apart. Electric field strength, E is the force exerted on unit charge at the point in question [10]. At distance r from charge q, E = q / 4π ⋅ ε 0 ⋅ r 2 (k) Solvent Effects An important topic in Chemistry concerns the effect of solvents on ion-ion interactions. Here we assume that solvents are characterised by their relative permittivities, εr. In a solvent the pairwise cation-anion interaction energy is given by equation (l) . U ij / J = − z i ⋅ z j e 2 / 4π ⋅ ε 0 ⋅ ε r ⋅ r U ij / J mol -1 = − z i ⋅ z j ⋅ N A ⋅ e 2 / 4π ⋅ ε 0 ⋅ ε r ⋅ r (l ) (m) As commented above, εr is always greater than unity. Hence for a given system at fixed distance apart r, Uij increases (becomes less negative) with increase in εr. With increase in εr, the ions are increasingly insulated and so at given distance r the stabilisation of the cation-anion pair is less marked. Molecular Dipole Moments A given molecule comprises an assembly of positive and negative charges. Consider a point 0, distance r from this assembly. We are concerned with the electric field strength at point 0, a short distance from the dipole moment. In the previous section we assumed that this assembly is simply characterised by the electric charge (i.e. z j ⋅ e for ion j). However, in those cases where the overall charge is zero, a measurable electric field is detected at point 0. In 1912 Peter Debye showed that this field could be accounted for as a first approximation by characterising a molecule by its dipole moment. In the next approximation the electric field at 0 can also be accounted by an additional contribution from a distribution of charges within a molecule called a quadrupole, and in the next approximation by an additional contribution from a distribution called an octupole [11]. In a homonuclear diatomic molecule such as H2 and Cl2, the positive nuclei are embedded in charge clouds describing the distribution of negatively charged electrons. For such molecules the "centres" of positive charges and negative charges coincide. But for the molecule HCl the electron distribution favours the more electronegative chlorine atom. Hence the centres of positive and negative charges, magnitude +q and -q, are separated by a dipole length l . The molecule has a dipole moment, a characteristic and permanent property of an HCI molecule. The (molecular) dipole moment µ is given by the product ‘ q ⋅ l '. A dipole moment has both magnitude and direction; it is a vector [12]. Footnotes [1] The classic reference in this subject is: J. O. Hirschfelder, C. F. Curtiss and R. B. Bird, Molecular Theory of Gases and Liquids, Wiley, New York 1954; corrected printing ,1964. [2] The ampere is that constant current flowing in two parallel straight conductors, having negligible cross section, one metre apart in vacuo which produces a force between each metre of length equal to 2 x 10-7 N. [3] When a current of one A flows for one second, the total charge passed is one coulomb. In practice, a current of 1 A is very high and the common unit is milliampere (symbol: mA). The starter motor in a conventional car requires a peak current of around 30 A. Electric charge on a single proton, e = 1.602 x 10-19 C. Faraday, F = N A ⋅ e = 9.649 x 10 4 C mol -1 [4] Just to keep up with the way the units are developing we note: electric current electric potential gradient coulomb C = A s volt V = J A-1 s-1 = J C-1 (J = joule). Thus volt expressed as J C-1 is energy per coulomb of electric charge passed. This link between electric potential and energy is crucial. electrical resistance ohm Ω = V A-1 Ohm's Law is a phenomenological law. [5] Continuing our concern for units. electrical capacitance: unit = farad F ≡ A s V-1 electric permittivity ε ; unit = F m-1 electric permittivity of a vacuum, ε0 = 8.854 x 10 -12 F m -1 relative permittivity ε r = ε / ε 0; unit = 1 Older literature calls εr, the "dielectric constant". But this property is not a constant for a given substance such as water ( l ). Thus ε and εr depend on both temperature and pressure [6]; ε and ε r for a given liquid depend on electric field strength and frequency of AC current applied to the capacitance. [7] The quantity (4 ⋅ π ⋅ ε 0 ⋅ 10 −7 ) −1 / 2 equals 2.998 x 10 8 m s -1 which is the speed of light. [8] We check the units. If X is a force, the unit for X is newton (symbol N). Then the right-hand side should simplify to the same unit. Electric charge is expressed in C [= A s]; ε0 has units of F m -1 [= A s V-1 m -1]. Then X = [C].[C]/[1].[1].[A s V-1 m-1]. [m]2 But [V] = [J A-1 s-1] Then X = [A2 s2]/ [A s J -1 A s m] = [J m -1l = [N] [9] We check that our units are correct. If Uij is an energy expressed in joules, the terms on the right-hand side should reduce to joules. U ij = [A s] ⋅ [A s]/[1] ⋅ [1] ⋅ [A s V -1 m -1 ] ⋅ [m] = [A 2 s 2 ] /[ A s J -1 A s] = [J] [10] E = [C] /[1] ⋅ [1] ⋅ [A s V -1 m -1 ] ⋅ [m 2 ] = [A s]/[A s V -1 m] = [V m -1 ] = [J A -1 s −1 m -1 ] = [J m −1 ] /[A s] = [N C -1 ] Thus electric field strength is expressed in V m-1 or N C-1; the latter is clearly a force per unit charge. [11] The classic text is:- P. Debye, Polar Molecules, Chemical Catalog Co., New York 1929 (available as Dover paperback). We do not consider here interactions involving quadrupoles, octupoles, etc. These molecular properties are reviewed by A. D. Buckingham, Quart. Rev., 1959, 13, 183. [12] Thus Dipole moment, µ = q ⋅ l = [C] ⋅ [m ] µ = [C m], coulomb metre . Dipole moments are normally quoted using the unit, debye. [The unit is named in honour of Peter Debye.] 1 D = 3.336 x 10 -30 C m.