* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Management of the postoperative Fontan patient
Survey
Document related concepts
Remote ischemic conditioning wikipedia , lookup
Heart failure wikipedia , lookup
Coronary artery disease wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Cardiac surgery wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Atrial septal defect wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
Transcript
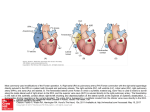
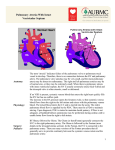
Progress in Pediatric Cardiology 17 (2003) 73–79 Management of the postoperative Fontan patient Deborah R. Gersony*, Welton M. Gersony College of Physicians and Surgeons, Columbia University, 630 W. 168th Street PH-3-343, New York, NY 10032, USA Abstract The purpose of the Fontan operation is to effectively normalize the circulation pathway in patients with a single ventricle. Pulmonary blood flow is established by a direct systemic venous connection to the pulmonary artery without an interposed ventricular chamber; the single ventricle provides systemic blood flow. The modern Fontan procedure consists of a direct superior vena cava to pulmonary artery anastomosis with the inferior vena cava blood reaching the pulmonary artery by means of an internal tunnel through the right atrium or an extra cardiac conduit from the inferior vena cava to the pulmonary artery. The Fontan operation is performed in patients from late infancy to adulthood, and is now one of the most common surgical procedures for congenital heart disease over the age of two years. Excellent palliation, and increased life expectancy is expected for most patients. However, over time, complications may occur and they require careful diagnostic evaluation and specific management. Some manifestations may appear in patients with any form of single ventricle, whereas others are specifically related to the morphology of the original single ventricle anatomy. Potential late complications include: obstruction in the systemic venous pathway, competitive pulmonary collateral blood flow, significant A–V valvar insufficiency, bulboventricular foramen obstruction, ventricular dysfunction, arrhythmia, protein losing enteropathy, and thromboembolism. This monograph addresses the clinical and laboratory assessment of patients who have had a Fontan operation, and discusses the various complications and management options. 䊚 2003 Elsevier Science Ireland Ltd. All rights reserved. Keywords: Single ventricle; Fontan procedure; Glenn operation 1. Management of the postoperative Fontan patient Prior to the development of the Fontan procedure, pulmonary blood flow in patients with single ventricle and pulmonary stenosis was surgically augmented by means of systemic to pulmonary artery shunts. These shunts improved life expectancy remarkably in the short term, but survival past the second decade remained unusual w1x. The complications of continued cyanosis, ventricular volume overload, and ventricular failure remained significant risks with these procedures. The concept that the right ventricle could be bypassed allowing systemic blood to enter the pulmonary artery directly was first established on experimental animals in 1970 w2x. A right atrial to pulmonary artery surgical procedure in humans with tricuspid atresia was introduced almost simultaneously by Fontan w3x and Kreutzer in 1971 w4x. Over the years, the indications for the Fontan operation gradually expanded to all patients with *Corresponding author. Tel.:q1-212-305-0629; fax: q1-212-3054429. E-mail address: [email protected] (D.R. Gersony). single ventricle physiology, and has become the most common operation performed for congenital heart disease after the age of two years. Originally, the procedure included anastomosis of the right atrium to the pulmonary artery directly (Fig. 1), with valves placed in the superior vena cava andyor inferior vena cava. It soon became clear that the valve placements, rather than being advantageous, resulted in obstruction of the lowpressure vena cava to pulmonary artery circulation. Furthermore, long-term follow-up of patients with the direct right atrial appendage to pulmonary artery anastomosis indicated that they were prone to late complications. These were most often related to turbulent flow within a markedly dilated right atrium that resulted in an energy loss state, and stagnant right sided blood flow. The patients developed exercise intolerance and other symptoms of low cardiac output, and had a predilection to cardiac arrhythmias associated with the dilated atrium. On the basis of this experience, the original Fontan operation has undergone numerous changes. In the modern era, the modified Fontan operation often includes an earlier bi-directional Glenn (SVC-PA anas- 1058-9813/03/$ - see front matter 䊚 2003 Elsevier Science Ireland Ltd. All rights reserved. PII: S 1 0 5 8 - 9 8 1 3 Ž 0 3 . 0 0 0 1 1 - 0 74 D.R. Gersony, W.M. Gersony / Progress in Pediatric Cardiology 17 (2003) 73–79 in the right sided circulation can cause significant obstruction, leading to symptoms such as peripheral edema and fatigue. Whether morphology of the single ventricle affects ventricular function over the long term remains unclear w6x. It may seem axiomatic that a single systemic left ventricle would be more likely to have preserved function in the long-term, as compared to a single right ventricle, but this has yet to be convincingly demonstrated. Late ventricular dysfunction over time appears to be significant in both groups w7x. However, tricuspid insufficiency in a patient with a systemic right ventricle may result in earlier heart failure then mitral incompetence in a single left ventricle. Adults with the Fontan circulation offer unique challenges in that they are prone to the long term complications of ventricular dysfunction and atrial arrhythmias w5,8x. Thus, careful monitoring of their functional status is mandatory. 2. Anatomy of single ventricle Patients who have had the Fontan procedure may have one of several forms of single ventricle. The Fontan operation was first designed for tricuspid atresia. In this defect, the tricuspid valve is absent, and the presence of Fig. 1. The classic Fontan operation is shown diagramatically in the context of a double inlet single left ventricle with a bulboventricular foramen and rudimentary RV outflow chamber. The right atrial appendage is sewn directly into the right pulmonary artery. The systemic venous circulation is separated from the pulmonary circulation by oversewing the right A–V valve and atrial communication. Ao: aorta; RA: right atrium; RV: right ventricle; SLV; single left ventricle. tomosis) or hemi-Fontan procedure. Large systemic to pulmonary artery shunts are avoided. The Fontan operation is completed later by an internal tunnel from the inferior vena cava through the right atrium to the pulmonary artery (Fig. 2). Many centers will utilize an extracardiac conduit from the inferior vena cava to the pulmonary artery to avoid manipulation and suturing in the right atrium (Fig. 3). Fenestration of the intracardiac or exracardiac conduit is often carried out to allow systemic venous blood to vent to the left atrium. This procedure potentially increases cardiac output postoperatively, although at the cost of lower arterial O2 saturation. Pleural effusions have been reported to be less prominent after fenestration. The Fontan operation is still considered to be a palliative procedure, although one which potentially will remain effective for decades. It has been established that the Fontan operation prolongs life expectancy in patients with single ventricle w5x. However, late complications are frequent. With direct communication from the vena cava system to the pulmonary artery without a right sided pumping chamber, even minimal gradients Fig. 2. The lateral tunnel Fontan is demonstrated in this illustration of a double inlet single left ventricle. A prior bidirectional Glenn anastomosis of the SVC to the right pulmonary artery is shown. For completion of the Fontan, the IVC is tunneled within the right atrium to the right pulmonary artery. D.R. Gersony, W.M. Gersony / Progress in Pediatric Cardiology 17 (2003) 73–79 75 3. Preoperative assessment Fig. 3. The extracardiac Fontan operation is shown in a patient with a double inlet single left ventricle. An external conduit from the IVC is connected to the right pulmonary artery after a prior bi-directional Glenn shunt. a large atrial communication is required whereby both systemic venous and pulmonary venous blood enters the left atrium, left ventricle, and subsequently the aorta. There is usually a restrictive ventricular septal defect, and an underdeveloped rudimentary right ventricle resulting in limited pulmonary blood flow. The double inlet left ventricle patient has both atrioventricular valves empty into a morphological left ventricle and only a rudimentary right ventricular outlet chamber exists. A bulbo-foramen (BVF) ‘VSD’ connects the left ventricle to the small right ventricular outlet, which in turn is connected to the transposed aorta. The BVF may be small, and this would result in associated subaortic stenosis. Hypoplastic left heart syndrome (HLHS) is also a form of single ventricle. This anomaly is initially palliated by the Norwood procedure in which the pulmonary artery and narrow ascending aorta are anastomosed; the pulmonary artery serves as the aorta and the right ventricle remains the systemic ventricle. An arterial-PA or right ventricular-PA shunt supplies pulmonary blood flow. HLHS patients eventually undergo a Fontan procedure, after a second stage bi-directional SVC-PA shunt. Less common forms of univentricular hearts include unbalanced atrioventricular canal defects as well as the Holmes heart in which the ventricular septum is absent, but the intracardiac anatomy is normal. The success of the Fontan procedure is related to the selection criteria. The ideal low risk candidate will have normal pulmonary arteries with low pulmonary pressure and resistance. There would be no significant atrioventricular regurgitation and the single ventricular function would be normal with a low ventricular end-diastolic pressure w7x. Originally the requirements for potential Fontan candidates were strict; most patients who underwent the procedure had tricuspid atresia and excellent hemodynamics. Over the past decade it has become clear that the early selection criteria were too inflexible and excluded a significant number of patients who could potentially benefit from the procedure. Non-fulfillment of some of the original criteria are now regarded as risk factors, rather than absolute contraindications w9x. It is important to identify the potential anatomical and functional risks for they may need to be addressed at preoperative catheterization or at the time of surgery. Excess collateral pulmonary artery blood flow from systemic arterial collaterals or a previous systemic arterial shunt may result in competitive pulmonary blood flow and increased PA pressure after a Fontan operation. Coiling of intermediate and large collaterals andyor persistent surgical systemic arterial shunts can usually be achieved during preoperative catheterization w11x. Distortion of the pulmonary arteries as a result of earlier shunts or PA banding, often requires pulmonary arterioplasty as a component of Fontan surgery. Anomalies of the systemic or pulmonary venous system have been incorporated into the Fontan circulation with minimal additional risk w10x. Significant atrioventricular valve regurgitation is poorly tolerated by patients with a Fontan circulation. Elevated pulmonary venous pressure, inevitably results in a mandatory increase in pressure in the Fontan systemic venous circulation in order to perfuse the lungs. In these cases, the clinical findings of low cardiac output syndrome, peripheral edema, fatigue and abdominal discomfort due to a congested liver may become manifest. Patients with atrioventricular valve regurgitation can have the insufficient valve surgically repaired or replaced at the time of the Fontan operation. Preoperative A–V insufficiency is considered an important risk factor, although satisfactory surgical results have been reported w12,13x. If there is ventricular outflow obstruction, enlargement of the bulboventricular foramen or a Damus–Kaye–Stansel (DKS) procedure to merge the great arteries may be required. It is important to note that if there is mild morphologic obstruction to systemic outflow, the gradients may progress over time. Even a moderate gradient may be poorly tolerated in the Fontan circulation, if ventricular function is affected. An appropriate strategy to relieve obstruction to systemic blood 76 D.R. Gersony, W.M. Gersony / Progress in Pediatric Cardiology 17 (2003) 73–79 flow should be considered concomitant with the Fontan procedure, but reoperation may be necessary if obstruction progresses over time. Both enlargement of BVF and the DKS procedure have been reported to be successful in this regard w14,15x. Significant pulmonary hypertension is an absolute contraindication to Fontan palliation. However, mild pulmonary hypertension in the presence of increased flow from a systemic shunt or in a patient after a less than optimal pulmonary artery banding is not a contraindication for a Fontan procedure. 4. The postoperative adult patient Most post-operative Fontan patients are asymptomatic, leading active quality lives. However, late symptoms in the adult are not uncommon. These most often consist of diminished exercise tolerance, fatigue, and palpitations. Obstruction in the Fontan circulation, ventricular dysfunction, increased A–V valve insufficiency or an arrhythmia most often present as fatigue or dyspnea on exertion. Complaints of lower extremity or abdominal swelling are important to investigate as they may represent the relatively uncommon but serious complication of protein losing enteropathy (PLE). A patient with an uncomplicated course will have an unremarkable physical examination. The cardiovascular examination will demonstrate no significant murmurs and a single S2. The jugular venous pressure is often elevated, reflecting high venous pressure in the Fontan circulation. In patients with significant hemodynamic abnormalities (e.g. A–V valve insufficiency, subaortic stenosis, significant pulmonary collateral blood flow, etc.), classic physical findings will emerge. For example, a loud systolic ejection murmur at the base could suggest BVF obstruction; a left lower sternal border or apical pansystolic murmur is usually associated with A–V valve insufficiency. A specific diagnosis can be made on the basis of laboratory studies (e.g. Chest X-ray, ECG, 2D echo, catheterization, Holter studies, O2 sat. etc.) The postoperative Fontan patient should have normal or near normal oxygen saturation. If significant cyanosis persists, it could have several different etiologies. If there was an atrial fenestration, a persistent right-to-left shunt may be present. Fenestrations may be closed by a device in the cardiac catheterization laboratory to both correct cyanosis and prevent paradoxical embolization. Before such a device is placed, temporarily balloon occlusion of the fenestration may be useful in order to determine the effect of closure on hemodynamics. If aortic pressure remains stable and right atrial pressure does not increase significantly, it is considered appropriate to close the fenestration. A 10 year follow-up study by Goff and colleagues of 154 patients, suggests that device closure of the Fontan fenestration is safe, and results in improved oxygenation and a reduced need for anticongestive medication w16x. The incidence of death or significant clinical decompensation in this study was rare (1.3 and 3.2%, respectively). This paper did not address the issue as to whether all Fontan patients should have an atrial fenestration as part of the original operation. With the advent of external conduits, some centers are carrying out fewer fenestrations w17x. Another etiology of persistent cyanosis in a Fontan patient is a leak of the IVC to right pulmonary artery baffle (e.g. an unintentional fenestration). These baffle leaks are often small and do not cause significant cyanosis, but if necessary, they can be closed by transcatheter device with the same preclosure techniques to observe and evaluate the hemodynamic effects as with fenestrations. Occasionally, a persistent left SVC may cause significant right-to-left shunting by draining venous blood into the coronary sinus or directly into the left atrium. Finally, intrapulmonary shunting through AV fistulae can be encountered in patients who have had either a bi-directional or, more commonly, a classic Glenn anastomosis w18x. 5. Complications 5.1. Arrhythmia Late atrial tachyarrhythmias are frequently seen in post-Fontan population, especially in patients who have had a classic atrial-pulmonary artery anastomosis. Ghai and colleagues w19x reviewed the outcomes of 94 consecutive patients who underwent the Fontan procedure between 1977 and 1994. Forty-one percent of patients developed sustained atrial tachycardia. The authors found that many were likely to exhibit atrial enlargement and have a right atrial thrombus. A higher frequency of significant systemic valve regurgitation was also demonstrated and ventricular dysfunction was not uncommon. It remains to be seen whether the extracardiac Fontan, which requires less direct manipulation or suturing of atrial tissue, may result in a decrease in the incidence of atrial arrhythmias. Preliminary early and mid-term reports have been encouraging, but not definitive w20x. Given the long-term risks of ventricular dysfunction and thrombus formation, it is important to reinstitute sinus rhythm in patients with sustained atrial arrhythmia. In most cases, anticoagulation should be given for 4–6 weeks followed by cardioversion. Unfortunately, recurrence is common and chronic antiarrhythmic drug treatment is often needed to maintain sinus rhythm. Antiarrhythmic therapy may be complicated by limited drug efficacy and underlying abnormalities of the sinus node and AV conduction system. Choice of drug options are influenced by the concomitant presence of ventricular dysfunction. Amiodarone has been increasingly D.R. Gersony, W.M. Gersony / Progress in Pediatric Cardiology 17 (2003) 73–79 used in patients with poor function and refractory arrhythmias w18x. Radiofrequency ablation is an available option for recurrent tachyarryhthmias. Mapping studies have demonstrated that atrial reentry tachycardias after the Fontan operation are significantly different from typical atrial flutter. The arrhythmias encountered in Fontan patients occur in areas of non-conductive atriotomy scars or surrounding prosthetic material. Reentrant activation occurs in close proximity to these regions and adjacent anatomic landmarks such as the SVC or IVC, and the atrioventricular annulus w21x. Electrophysiology studies can identify these areas. These zones may be amenable to interruption with a line of block between surgical and anatomical barriers w22,23x. However, long-term success with radiofrequency ablation in the catheterization laboratory has been variable in terms of maintaining long-term sinus rhythm without recurrent atrial tachycardia. In severe cases, a surgical MAZE procedure with epicardiac DDD pacing can be carried out in conjunction with revising the classic RA–PA Fontan to a lateral tunnel or extra cardiac conduit. There have been reports of improved functional class and lowered incidence of recurrent arrhythmias with this approach w24x. For patients with sinus node dysfunction or heart block, epicardial pacing is a viable option and results are similar to patients with biventricular physiology w25x. 5.2. Protein-losing enteropathy (PLE) An infrequent but serious late complication of the Fontan procedure is PLE. The findings include hypoalbuminenia, ascites, pleural effusions, diarrhea, malaise and lymphocytopenia. The prevalence of this complication is reported to range from 1.5 to 11% w26x. Although the etiology of PLE is not known, it has been assumed that it is related to the elevated systemic venous pressures of the Fontan circuit. A recent retrospective analysis of 398 Fontan patients attempted to identify risk factors for the development of PLE w27x. Potential risk factors included postoperative systemic venous pressure, cardiopulmonary bypass time, single right ventricle anatomy, gender, age at Fontan operation, anomalous venous drainage, atrioventricular valve regurgitation, pulmonary artery distortion, and pre-Fontan measurements of systemic venous pressure and pulmonary artery resistance. In this study, longer bypass time and single right ventricular anatomy were the only peri-operative risk factors that were associated with the development of PLE. The postoperative systemic venous pressure was not significantly correlated with this complication. The authors speculated that peri-operative cardiac injury may serve as a potentiating factoring in the development of PLE. At the onset of PLE, any evidence of abnormal hemodynamics or obstruction in the Fontan circuit should be investigated by cardiac catheterization. 77 The management of PLE is challenging. Symptomatic treatment includes, diuretics, supplemental albumin infusion, and dietary modifications to high protein and high medium-chain triglyceride intake and some benefit has been reported w28,29x. There are sporadic case reports of improvement with oral steroids and heparin, which are thought to stabilize intestinal cell membranes w30,31x. For refractory cases, attempts to improve hemodynamics and lower the pressure in the systemic venous system have been undertaken with fenestration of the systemic venous baffle, and some positive results have been forthcoming w32x. Under extreme circumstances, in unresponsive patients, cardiac transplantation is an effective treatment w33–35x. 5.3. Ventricular dysfunction Several studies have demonstrated decreased systolic ventricular performance over time after a Fontan operation. A recent prospective study evaluated the incidence of heart failure and ventricular dysfunction in patients with systemic right or single ventricles under various circumstances w7x. The highest risk group were patients who were status post the Fontan procedure, as compared to patients with congenitally corrected transposition or post Mustard repair of transposition of the great arteries. The incidence of documented heart failure was 40% in this group of patients w7x. The reason for the high incidence of progessive ventricular dysfunction in the Fontan group over time is not clear. Cheung et al. w36x has shown evidence of progressive diastolic dysfunction in pediatric patients, and speculate that abnormal ventricular relaxation may play a role in the development of later systolic dysfunction. Although little data are available regarding optimal medical treatment for ventricular dysfunction after the Fontan procedure, standard therapy includes diuretics, digoxin and afterload reduction with ACE-inhibitors. The role of ACE-inhibitors in patients without symptoms of heart failure is not yet defined w37x. For patients with a classic RA–PA Fontan, who have severe symptomatic ventricular dysfunction despite maximal medical therapy, conversion to a lateral tunnel or external conduit has been effective in increasing cardiac output and alleviating symptoms. The operation is often done in conjunction with a MAZE procedure since chronic atrial arrhythmias are frequently associated with Fontan failure. Heart transplantation offers a mode of treatment when others have been exhausted, and good results are reported for the majority of patients w38x. 5.4. Thromboembolism Thromboembolic events are a not infrequent complication of Fontan patients. The reported rate of significant thrombotic events has been reported to be as high as 78 D.R. Gersony, W.M. Gersony / Progress in Pediatric Cardiology 17 (2003) 73–79 33% w38,39x. The etiology of embolic events has been attributed to atrial arrhythmias, sluggish blood flow, right-to-left shunts, and hypercoaguable states. The role of anticoagulation for this group of patients is not clear. The use of warfarin for prevention of thrombotic events in patients with dense spontaneous echocontrast in the right atrium is often recommended. Although antiplatelet therapy is not generally recognized as being effective w39x, Jacobs and colleagues recently found no evidence of either thromboembolic events or asymptomatic thrombus in 72 patients treated with 81 mg of aspirin and followed for a mean of 40 months w40x. It should be noted that in this study, transthoracic echocardiography was the surveillance technique used, and this is a less sensitive test compared to a transesophageal echocardiography in the detection of intracardiac thrombus. 5.5. Pregnancy During pregnancy cardiac output increases by 30– 40% and intravascular volume by 50%. Stroke volume and heart rate increase, and systemic and pulmonary resistance decreases. As a result, there are significantly increased cardiac volume requirements. Fontan patients have a limited ability to increase cardiac output and have elevated venous pressures. Thus, there are concerns regarding the hemodynamic effect of pregnancy on the Fontan patient. Nevertheless, successful pregnancies in patients with excellent pre-pregnancy Fontan hemodynamics have been reported w41,42x. No reports of maternal deaths have appeared, but there is an increased incidence of spontaneous abortion and pre-term labor. There also is an increased risk of atrial arrhythmia and heart failure during pregnancy. Whether there are permanent changes in maternal hemodynamics is unknown. On balance, given the risks for mother and fetus, most cardiologists discourage pregnancy for Fontan patients. 6. Conclusion The modern Fontan operation remains a palliative procedure for single ventricle patients. Mortality and morbidity are low. Outcomes are more favorable than for those patients who have had systemic to pulmonary artery shunts alone. Associated defects such as bulboventricluar foramen obstruction and atrioventricular regurgitation should be treated concommitantly at the time of the Fontan operation. However, there remain significant long-term complications including arrhythmias, A–V valve insufficiency, ventricular dysfunction, thromboembolism and protein-losing enteropathy. Symptomatic patients with classic Fontans can be converted to lateral tunnel or external conduits with MAZE procedures to treat or prevent atrial arrhythmias. Cardiac transplantation represents a final option for Fontan patients with intractable low cardiac output. References w1x Dick M, Fyler DC, Nadas AS. Tricuspid atresia: clinical course in 101 patients. Am J Cardiol 1975;36:327. w2x Visudh-Arom K, Miller ID, Castaneda AR. Total cardiopulmonary bypass in puppies: pulmonary studies. Surgery 1970;68(5):878 –883 (Nov). w3x Fontan F, Mounicot F, Baudet E, Simonneau J, Gordo J, Gouffrant J. Correction de l’atresie tricuspidienne: rapport de deux cas ‘corriques’ par l’utilization dune technique chirurqicale nouvelle. Ann Chir Thorac Cariovasc 1971;10:39 –47. w4x Kreutzer G, Galindez E, Bono H, De Palma C, Laura JP. An operation for the correction of tricuspid atresia. J Thorac Cardiovasc Surg 1973;66(4):613 –621 (Oct). w5x Veldtman GR, Nishimoto A, Siu S, Freeman M, Fredriksen PM, Gatzoulis MA, et al. The Fontan procedure in adults. Heart 2001;86:330 –335. w6x Gaynor JW, Bridges ND, Cohen MI, Mahle WT, Decampli WM, Steven JM, et al. Spray TL Predictors of outcome after the Fontan operation: is hypoplastic left heart syndrome still a risk factor? J Thorac Cardiovasc Surg 2002;123(2):237 –245 (Feb). w7x Piran S, Veldtman G, Siu S, Webb G, Liu P. Heart failure and ventricular dysfunction in patients with single ventricle or systemic right ventricles. Circulation 2002;105:1189 –1194. w8x Gates RN, Laks H, Drinkwater DC Jr, Lam L, Blitz A, Child JS, et al. The Fontan procedure in adults. Ann Thorac Surg 1997;63:1085 –1090. w9x Choussat A, Fontan F, Besse P, Vallot F, Chauve A, Bricaud H. Selection criteria for Fontan’s procedure. In: Anderson RH, Shinebourne EA, editors. Paediatric Cardiology. Edinburgh: Churchill Livingstone, 1977. p. 559 –566. w10x Michielon G, Gharagozloo F, Julsrud PR, Danielson GK, Puga FJ. Modified Fontan operation in the presence of anomalies of systemic and pulmonary venous connection. Circulation 1993;88 (5 Pt 2):I141 –I148 (Nov). w11x Kanter KR, Vincent RN. Management of aortopulmonary collateral arteries in Fontan patients: Occlusion improves clinical outcome. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2002;5(1):48 –54 (Jan). w12x Cetta F, Feldt RH, O’Leary PW, Mair DD, Warnes CA, Driscoll DJ, et al. Improved early morbidity and mortality after Fontan operation: The Mayo clinic experience, 1987 to 1992. J Am Coll Cardiol 1996;28(2):480 –486 (Aug). w13x Imai Y, Takanashi Y, Hoshino S, Terada M, Aoki M, Ohta J. Modified Fontan procedure in ninety-nine cases of atrioventricular valve regurgitation. J Thorac Cardiovasc Surg 1997;113(2):262 –268 (Feb). w14x Hiramatsu T, Imai Y, Kurosawa H, Takanashi Y, Aoki M, Shinoka T, et al. Midterm results of surgical treatment of systemic ventricular outflow obstruction in Fontan patients. Ann Thorac Surg 2002;73(3):855 –860 (Mar). w15x Hsu DT, Quaegebeur JM, Ing FF, Selber EJ, Lamour JM, Gersony WM. Outcome after the single-stage, non-fenestrated Fontan procedure. Circulation 1997 Nov 4;96(9 Suppl):II335–40. w16x Goff DA, Blume ED, Gauvreau K, Mayer JE, Lock JE, Jenkins KJ. Clinical outcome of fenestrated Fontan patients after closure: the first 10 years. Circulation 2000;102(17):2094 – 2099 (Oct 24). w17x Thompson LD, Petrossian E, McElhinney DB, Abrikosova NA, Moore P, Reddy VM, et al. Is it necessary to routinely fenestrate an extracardiac Fontan? J Am Coll Cardiol Aug 1999;34(2):539 –544. w18x Gersony WM, Rosenbaum MS. Congenital Heart Disease in the Adult. New York, Chicago, San Francisco, Lisbon, London, D.R. Gersony, W.M. Gersony / Progress in Pediatric Cardiology 17 (2003) 73–79 w19x w20x w21x w22x w23x w24x w25x w26x w27x w28x w29x Madrid, Mexico City, Milan, New Delhi, San Juan, Seoul, Singapore, Sydney, Toronto: McGraw Hill Medical Publishing Division, 2002. Ghai A, Harris L, Harrison DA, Webb GD, Siu SC. Outcomes of late atrial tachyarrhythmias in adults after the Fontan operation. J Am Coll Cardiol 2001;37(2):585 –592 (Feb). Azakie A, McCrindle BW, Van Arsdell G, Benson LN, Coles J, Hamilton R, et al. Extracardiac conduit vs. lateral tunnel cavopulmonary connections at a single institution: impact on outcomes. J Thorac Cardiovasc Surg 2001;122(6):1219 –1228 (Dec). Collins KK, Love BA, Walsh EP, Saul JP, Epstein MR, Triedman JK. Location of acutely successful radiofrequency catheter ablation of intraatrial reentrant tachycardia in patients with congenital heart disease. Am J Cardiol 2000;86(9):969 – 974 (Nov 1). Baker BM, Lindsay BD, Bromberg BI, Frazier DW, Cain ME, Smith JM. Catheter ablation of clinical intraatrial reentrant tachycardias resulting from previous atrial surgery: localizing and transecting the critical isthmus. J Am Coll Cardiol 1996;28(2):411 –417 (Aug). Triedman JK, Alexander ME, Berul CI, Bevilacqua LM, Walsh EP. Electroanatomic mapping of entrained and exit zones in patients with repaired congenital heart disease and intra-atrial reentrant tachycardia. Circulation 2001;103(16):2060 –2065 (Apr 24). Mavroudis C, Backer CL, Deal BJ, Johnsrude C, Strasburger J. Total cavopulmonary conversion and maze procedure for patients with failure of the Fontan operation. J Thorac Cardiovasc Surg 2001;122(5):863 –871 (Nov). Cohen MI, Vetter VL, Wernovsky G, Bush DM, Gaynor JW, Iyer VR, et al. Epicardial pacemaker implantation and followup in patients with a single ventricle after the Fontan operation. J Thorac Cardiovasc Surg 2001;121(4):804 –811 (Apr). Mertens L, Hagler DJ, Sauer U, Somerville J, Gewillig M. Protein-losing enteropathy after the Fontan operation: an international multicenter study. PLE study group. J Thorac Cardiovasc Surg 1998;115:1063 –1073. Powell AJ, Gauvreau K, Jenkins KJ, Blume ED, Mayer JE, Lock JE. Perioperative risk factors for development of proteinlosing enteropathy following a Fontan procedure. Am J Cardiol 2001;88(10):1206 –1209 (Nov 15). Rychik J, Spray TL. Strategies to treat protein-losing enteropathy. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2002;5(1):3 –11 (Jan). Guariso G, Cerutti A, Moreolo GS, Milanesi O. Protein-losing enteropathy after Fontan operation: treatment with elementary diet in one case. Pediatr Cardiol 2000;21(3):292 (May–Jun). 79 w30x Facchini M, Guldenschuh I, Turina J, Jenni R, Schalcher C, Attenhofer Jost CH. Resolution of protein-losing enteropathy with standard high molecular heparin and urokinase after Fontan repair in a patient with tricuspid atresia. J Cardiovasc Surg (Torino) 2000;41(4):567 –570 (Aug). w31x Zellers TM, Brown K. Protein-losing enteropathy after the modified Fontan operation: oral prednisone treatment with biopsy and laboratory proved improvement. Pediatr Cardiol 1996;17(2):115 –117 (Mar–Apr). w32x Lemes V, Murphy AM, Osterman FA, Laschinger JC, Kan JS. Fenestration of extracardiac Fontan and reversal of proteinlosing enteropathy: case report. Pediatr Cardiol 1998;19:355 – 357. w33x Holmgren D, Berggren H, Wahlander H, Hallberg M, Myrdal U. ‘Reversal of protein-losing enteropathy in a child with Fontan circulation is correlated with central venous pressure after heart transplantation.’ Pediatr Transplant 2001 Apr;5(2):135–7. w34x Therrien J, Webb GD, Gatzoulis MA. Reversal of protein losing enteropathy with prednisone in adults with modified Fontan operations: long term palliation or bridge to cardiac transplantation? Heart 1999;82(2):241 –243 (Aug). w35x Sierra C, Calleja F, Picazo B, Martinez-Valverde A. Proteinlosing enteropathy secondary to Fontan procedure resolved after cardiac transplantation. J Pediatr Gastroenterol Nutr 1997;24(2):229 –230 (Feb). w36x Cheung YF, Penny DJ, Redington AN. Serial assessment of ventricular diastolic function after the Fontan procedure. Heart 2000;83(4):420 –424 (Apr). w37x Kouatli A, Garcia JA, Zellers TM, Weinstein EM, Mahony L. Enalapril does not enhance exercise capacity in patients after Fontan procedure. Circulation 1997;96:1507 –1512. w38x Hsu DT, Quaegebeur JM, Michler RE, Smith CR, Rose EA, Kichuk MR, et al. Heart transplantation in children with congenital heart disease. JACC 1995;26(3):743 –949. w39x Ravn HB, Hjortdal VE, Stenbog EV, Emmertsen K, Kromann O, Pedersen J, et al. Increased platelet reactivity and significant changes in coagulation markers after cavopulmonary connection. Heart 2001;85(1):61 –65 (Jan). w40x Jacobs ML, Pourmoghadam KK, Geary EM, Reyes AT, Madan N, McGrath LB, et al. Fontan’s operation: is aspirin enough? Is coumadin too much? Ann Thorac Surg 2002;73(1):64 –68 (Jan). w41x Ito M, Takagi N, Sugimoto S, Oosawa H, Abe T. Pregnancy after undergoing the Fontan procedure for a double outlet right ventricle: report of a case. Surg Today 2002;32(1):63 –65. w42x Canobbio MM, Mair DD, van der Velde M, Koos BJ. Pregnancy outcomes after the Fontan repair. J Am Coll Cardiol 1996;28(3):763 –767 (Sep).