* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Mitral Stenosis Etiology
Coronary artery disease wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Infective endocarditis wikipedia , lookup
Pericardial heart valves wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Cardiac surgery wikipedia , lookup
Artificial heart valve wikipedia , lookup
Aortic stenosis wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Atrial fibrillation wikipedia , lookup
Rheumatic fever wikipedia , lookup
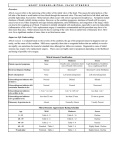
Mitral Stenosis Lori B. Heller, MD Clinical Instructor University of Washington Medical Center Physicians Anesthesia Service Swedish Medical Center Seattle, WA Etiology In the vast majority of cases, mitral stenosis is caused by rheumatic involvement of the mitral valve 1,2 although less than 70% of patients report a history of rheumatic fever3,4 . In a surgical pathology series of 452 patients with MS seen at the Mayo Clinic in 1987, 99 percent had post‐ inflammatory disease that was presumed to be rheumatic in origin. 3 Rheumatic Heart Disease is the most common cause of MS While the overall incidence of rheumatic fever and subsequent rheumatic mitral valve disease has become much less common in the United States, it still remains the most common cause of mitral stenosis. Another study of 1051 consecutive patients presenting for surgical correction of MS determined the etiology was rheumatic in 77 percent and may have been higher if not for the 15 percent of cases in which the etiology was not classified. 2 Infective endocarditis and mitral annular calcification accounted for 3.3 and 2.7 percent of cases, respectively. Other etiologies such as congenital malformation, systemic lupus erythematosus, carcinoid heart disease, Less Common Causes of MS: endomyocardial fibrosis, and rheumatoid arthritis were implicated in less than 1 percent of cases. Infective Endocarditis Mitral Annular Calcifications Congenital SLE Carcinoid While the US prevalence of RHD has sharply declined over the past 40 years, developing countries continue to have a significant prevalence. It is estimated that 15.6 million people suffer from rheumatic heart disease worldwide, with approximately 282,000 new cases and 233,000 related deaths each year.5 Pathophysiology and Course of Disease Patients with MS typically present more than 20 years after an episode of rheumatic fever. It is unclear if it is the initial infection combined with continued turbulent flow over the valve or if there is a smoldering rheumatic process that continues. Progressive thickening, scarring, and calcification of the mitral leaflets and chordae are the hallmark of RHD. Fusion of the commissures and chordae also cause a decrease the orifice size. This obstruction results in the development of a pressure gradient across the valve in diastole and causes an elevation in left atrial and pulmonary venous pressures. Elevated left atrial pressures then lead to left atrial enlargement, predisposing the patient to atrial fibrillation and arterial thromboembolism. Elevated pulmonary venous pressure results in pulmonary congestion and pulmonary edema. In advanced mitral stenosis, patients develop pulmonary hypertension and right‐sided heart failure.6 Secondary Findings LAE Afib Pulmonary Edema PHTN Right Heart Failure MVA Normal 4‐‐6 cm2 Mild Stenosis 1.6‐2.0 cm m2 Moderate SStenosis 1.1‐1 1.5 cm2 Severe Sten nosis < 1.0 cm m2 2D exam Initial evalu uation of the mitral valve sshould begin with 2D inspeection of the leaflets. Rheumatic mitrral stenosis is characterized by thickeneed, calcified leaflets witth obstruction n of inflow intto the left ventricle. In rheeumatic heartt disease, the e thickening b begins at the leaflet tips and extends down the commissures toward the e remaining pportions of th he leaflets. Sin nce leaflet mo otion is restricted d mostly at th he leaflet tipss, the leaflets have a characteristic dom ming appearance e in diastole ((hockey stick deformity). TThe posterior leaflet is ofteen immobile. TThe entire miitral valvular aapparatus is iinvolved in th his pathologicc Anterrior leaflet. Hock key process as t the chordae b become thick kened, shorte ened, and ofte en fused‐ stick deformity therefore ccontributing to the restrictted leaflet mo ovement. Long Axis View w of the Left Ventrricle: The anteriorr leaflet appears thickkened and displayys diastolic domin ng or a Com mmissural fussion is an impportant feature to distingu uish rheumatiic hockey‐stick deformity which is characteristic o of RHD from degenerative MS. It alsoo serves to id dentify severee MS as comp plete fussion of both commissures uusually indicaates more advvanced diseasse. Thiis can be seen n in the ME viiews however is perhaps b best appreciaated in tthe TG basal SSAX view. Postteromedial Comm missure Anterrolateral Commisssure TG Basal SAX alloows inspection of booth com mmissures MS 2D characteristics c s: Diastolic c Doming of anterior a leaflett Restricted motion of posterior p leafllet Thickening begins at leaflet tips ordae Shortened, fused cho Commis ssural fusion Pressure Gradients Elevvation of the mean pressu ure gradient iis the hallmark of mitral sstenosis.7,9,10 TTo determinee, obtain the maxim mal velocity u using continuo ous wave Dopppler from th he mid‐esophageal imagess. Tracce the entire mitral valve sspectral profiile and the coomputer softw ware will inteegrate the velo ocities and caalculate the m mean gradientt. Mean Gradient: Mild < 5 mm Hgg Moderate 5‐12 m mm Hg Sevvere > 12 mm Hg CW profile of the MV inflow. Tracin ng over the entire spectral profile w will yield a mean mitral pressure gradient from thee machine softwarre Calcculation of M MVA The ere are severaal methods avvailable to determine MVA A, each with aadvantages an nd disadvantaages. It is best to use sseveral techniques for confirmation of ddisease and sseverity. nimetry Plan Mitral valve areaa can be meassured by plan nimetry in thee transgastricc basal short aaxis view. Thee ima age of the mittral valve is frozen in diastole and the opening is trraced. Since tthe mitral vallve is not flat (it formss a funnel shape) the imagiing plane shoould be moved back and fo orth in order tto e. This will bee the limiting aspect of thee ventricular determine the narrowest part of the valve inflo ow. Planimetryy of the mitral vvalve is performe ed in the basal short axis vie ew. The narrow west portion off the valve shou uld be traced. Plan nimetry Limittations: Exte ensive calcificcations can m make determin nation of the exact locatio on of the mitrral valve edgee difficult. Patientss with previous commissurotomies are also technicaally challengin ng. Caution not to ove er‐gain as this can lead to e error.10 Pressure Half Tim me: PHTT uses the rate of pressure e drop across the mitral vaalve as a meassure of mitrall severity. As the severity of the m mitral stenosiss increases, th he time it takkes the pressu ure between tthe left atrium m and d left ventricle e to equalize during diasto ole increases. Pressure halff time is the ttime it takes ffor the peak transmitral pressure e gradient to decrease by hhalf. When th he mitral valvve area is 1.0 cm2 it taakes 220 ms ffor the pressu ure gradient aacross the mittral valve to d drop to half itts original value. The erefore, 220 d divided by the e pressure half time will givve the mitral valve area in n cm2. MV VA = 220/PHT Nott all increased d PHT’s indicaate mitral sten nosis. Patientts with abnorrmal myocard dial relaxation n havve a prolonged d PHT, but the peak E velo ocity is not inccreased and iis usually low wer than 1 m/ssec. In p patients with atrial fibrillation, several ccycles should be averaged.. Occasionallyy, there are tw wo separate slopes of mitral velo ocity – a more e peaked initi al slope and aa second slop pe with a longger HT should be measured fro om the latter.. At faster heaart rates, the E wave and tthe A duration. The PH wavve begin to fu use and it mayy be difficult tto distinguishh the slope off the velocity profile. This m may be o overcome by decreasing th he sweep spe eed of the speectral profile.. Spectral Dopp pler pattern of m mitral inflow vvelocity. This caan be obtained ffrom any of the midesophageal LV views. Mostt TEE machines determine PHT have software packages that d when the E slope is trraced. To m measure Presssure Half Tim me: PH HT underestim mates MS seve erity In AI and impairred LV relaxatiion Optimize CW mitral inflow velocity 1. O 2. C Change sweep p speed to 10 00 mm/s 3. TTrace the slop pe of the E waave 4. TThe machine ssoftware packkage will provvide you withh PHT and MV VA manually: Calcculation of the PHT can alsso be done m 1. O Optimize CW mitral inflow velocity 2. C Change sweep p speed to 10 00 mm/s 3. M Measure the p peak E wave vvelocity, or VMAX M 4. VMAX ÷ 1.4 = V t1/2 5. D Draw vertical lines from E sslope to baseline at VMAX aand V t1/2 6. M Measure time e interval (T1/22) from VMAX tto V t1/2 7. M MVA = 220÷ TT1/2 Deceleration Time Alternatively, Deceleration time can be used to calculate MVA. Pressure half time is 0.29% of deceleration time. Therefore MVA = 760/DT PHT = 0.29 x DT PHT/Deceleration Time Limitations: Aortic Insufficiency and abnormal myocardial relaxation will underestimate the severity of MS by PHT. PHT has not been validated in the post CPB patients and has been found inaccurate in post‐commissurotomy patients due to its dependence on net chamber compliance. 8 Continuity Equation on and the conservation off energy statees: The continuity equatio LVOT (are ea) x LVOT (TV VI) = MV (areaa) x MV (TVI) LVOT areaa = left ventricular outflow w tract area LVOT (TVII) – left ventriicular outflow w tract time‐vvelocity integrral MV (TVI) = = mitral valve e time‐velocitty integral Mitral Vallve area can ttherefore be d determined w with the threee of the other variables in the equation n. 2 LVOT areaa is determine ed by calculattion of Πr (th he formula foor area of a circle), where rr is the ½ the diameter of the LVOT m measured in tthe aortic LAX X view and thhe respective VTI’s are obttained using spectral D Doppler. LVOTT VTI can be o obtained with h PWD in the deep transgaastric position n and the VTI of the MV ob btained in the e mid‐esophaageal position n using CWD. LVOTAREA x VTI = MVAREAA x VTI A Dooppler through LVO OT Diameter of the LVOT MVAREA = x CW through MV V Con ntinuity equation demonsstrated Associated Findiings The e increased left atrial presssure associate ed with mitraal stenosis leaads to left atrial enlargemeent, pid regurgitaation. Left atrial pulm monary hype ertension, right ventriculaar enlargemen nt and tricusp size e should be m measured in th he short axis vview of the aoortic valve, ass this view haas best been corrrelated with ttransthoracic images. Left atrial dilationn with low flo ow and high p pressure can lead to thrombus form mation and th he left atrium m and appenddage should b be inspected tthoroughly beefore and d after surgeryy. Regional w wall motion aabnormalitiess of the posteerior‐basal myyocardium in patients withoutt CAD occur w with severe rh heumatic mitrral stenosis and may indicated a mechaanical teth hering of the myocardium caused by a sscarred mitraal valve. All off these secondary findingss can help p confirm the e diagnosis off mitral stenossis and aid in the determin nation of seveerity. LA LAE RA Leftt atrial enlarggement Bowing inteeratrial septu um LA > > 39 mm indicative of LAEE Inte eratrial septu um bowing to o the right ind dicative of inccreased LAP Leftt atrial size sh hould be meaasured in the short axis vi ew of the AV V 12 Mean gradient ((mmHG) PHT T (msec) MV VA (cm2) Mitraal Stenosis Seeverity Mod derate MILD 6 100 0‐150 1.5 5‐2.0 6‐122 150‐‐200 1.0‐11.5 Severre >12 >220 0 <1.0 0 References 1. Olson LJ, Subramanian R, Ackermann DM, et al. Surgical pathology of the mitral valve: a study of 712 cases spanning 21 years. Mayo Clin Proc 1987; 62:22. 2. Horstkotte D, Niehues R, Strauer BE. Pathomorphological aspects, aetiology and natural history of acquired mitral valve stenosis. Eur Heart J 1991; 12 Suppl B:55. 3. WOOD P. An appreciation of mitral stenosis. I. Clinical features. Br Med J 1954; 1:1051. 4. ROWE JC, BLAND EF, SPRAGUE HB, WHITE PD. The course of mitral stenosis without surgery: ten‐ and twenty‐year perspectives. Ann Intern Med 1960; 52:741. 5. Carapetis JR, Steer AC, Mulholland EK, et al: The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005, 5: 685‐694. 6. Bruce C., Nishimura R. Valvular Heart Disease CLINICAL ASSESSMENT AND MANAGEMENT OF MITRAL STENOSIS. Cardiology Clinics. Volume 16 (3) August 1998 7. Zaroff J., Picard M Transesophageal Echocardiographic Evaluation of the Mitral and Tricuspid Valves. Cardiology Clinics. Volume 18 • Number 4 • November 2000 8. Thomas JD, Wilkins GT, Choong CY, et al. Inaccuracy of mitral pressure half‐time immediately after percutaneous mitral valvotomy. Dependence on transmitral gradient and left atrial and ventricular compliance. Circulation, Vol 78, 980‐993 9. Savino J Transesophageal Echocardiographic evaluation of Native Valvular Disease and Repair. Critical Care Clinics Vol 12(2). April 1996. 10. Porembka D. Transesophageal Echocardiography. Critical Care Clinics. Vol 12 (4) Oct 1996 11. Block M. Comparison of left atrial dimensions by transesophageal and transthoracic echocardiography. Journal of the American Society of Echocardiography. Volume 15 • Number 2 • February 2002 12. Block M, Hourigan L, Bellows W H. et al Comparison of left atrial dimensions by transesophageal and transthoracic echocardiography. J Am Soc Echocardiogr 2002. (15) 143–149.