* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download CHAPTER 3
Soil erosion wikipedia , lookup
Surface runoff wikipedia , lookup
Soil horizon wikipedia , lookup
Agroecology wikipedia , lookup
Arbuscular mycorrhiza wikipedia , lookup
Soil respiration wikipedia , lookup
Soil compaction (agriculture) wikipedia , lookup
Soil food web wikipedia , lookup
No-till farming wikipedia , lookup
Soil salinity control wikipedia , lookup
Human impact on the nitrogen cycle wikipedia , lookup
Crop rotation wikipedia , lookup
Soil contamination wikipedia , lookup
Terra preta wikipedia , lookup
Canadian system of soil classification wikipedia , lookup
Plant nutrition wikipedia , lookup
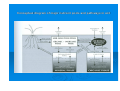
CHAPTER 3 FORMS OF SOIL N,P,K AND OTHER ELEMENTS NUTRIENT UPTAKE OF CROPS AND FACTORS INFLUENCING IT Prof. K. SÁRDI NUTRIENT MANAGEMENT FORMS OF SOIL NUTRIENTS Nutrients exist in numerous different forms (called as „nutrient pools” in the soil. These pools range from soluble to insoluble forms: soluble (= readily available ions in the soil solution), weakly bound (= adsorbed, easily exchangeable ions, also referred as available) strongly bound (= insoluble, precipitated compunds) IMPORTANT Readiliy available and weakly bound forms are in rapid equilibrium, insoluble/precipitated forms become available only over long time periods Conceptual diagram of major nutrient pools and pathways in soil Available nutrients = can be taken up directly by roots ions of readily water-soluble, inorganic compunds in the soil solution easily exchangeable by roots, as cations (K+ and NH4+ ) and anions (H2PO4-, NO3-) adsorbed (weakly bound) forms: • anions (e.g. phoshates, sulphates, nitrate) by organic colloid surfaces • cations (e.g. K+ and NH4+ ) adsorbed by clay minerals such as illites, montmorillonites, smectites etc. Sources of available soil nutrients: A.) natural sources Weathering of soil minerals Decomposition of plant residues, animal remains and soil microbes N fixation by symbiotic and other soil microorganisms (e.g. Rhisobium spp.) Deposition of nutrient-rich sediment from erosion and flooding Atmospheric origin: • • • Lightning discharges Acid rain at industrial regions Atmospheric deposition/dry B.) Sources under agricultural conditions Application of mineral fertilizers Application of manures, composts, sewage sludge and other organic amendments /wastes/ Application of industrial byproducts Application of ground rock powders, rock phosphate, basalt etc. Non-available nutrients = insoluble, strongly bound, fixed or precipitated forms Often called as the nutrient budget of the soil Strongly fixed cations (K+, NH4+, Mg2+, Ca2+) in interlayer sites of clay minerals Structural ions of soil minerals Nutrients taken up by soil microorganisms FACTORS INFLUENCING NUTRIENT AVAILABILITY AND UPTAKE FROM SOILS MECHANISMS OF ION TRANSPORT TO PLANT ROOTS 3 Mechanisms are known in which nutrients reach the root surface: - Root interception – physical contact resulted by root growth - Mass flow – transport to the root as a result of transpiration - Diffusion movement – resulted by differences in concentration - Rates of Root interception, Mass Flow and Diffusion in Ion Transport to Corn Roots (Havlin et al. 2005) Nutrient Root interception Mass Flow Diffusion movement Pecentages in Supply Nitrogen N 1 99 0 Phosphorus P 2 4 94 Potassium K 2 20 78 Calcium Ca 12 88 0 Magnesium Mg 27 73 0 Sulphur S 4 94 2 Nutrient uptake by plants (crops) - Nutrient uptake by roots – dominant in nutrition Nutrient uptake by leaves – additional in nutrition Nutrient absorption by roots is a process of ion exchange at the surface - Ion uptake of plant is characterized by the following: 1. Selectivity certain ions (elements) are taken up preferentially 2. Accumulation concentration of elements in the plant cell sap can be much higher than in the external solution 3. Genotype there are considerable differences among plant species in ion uptake characteristics passive part 1 Ion uptake active part 2 1. Movement of low-molecular-weight solutes (e.g. mostly ions, organic (amino) acids, sugars) from the external solution into the cell walls of roots this process is driven by diffusion or mass flow 2. Ion uptake is the movement of ions from the soil solution into the plant root against a concentration gradient. This is followed by the solute transport across membranes. The carrier and ion pump systems Carriers are the specific molecules to carry on ions across the cell membrane. Identification of carriers has not been completely determined yet. This process can be characterized by the following: - The ion is attached to a carrier The combined unit is transported from the root surface into the root The ion deposited inside the root with the carrier moving back across the cell membrane to repeat the process with another ion. Another concept An ion pumps that assist in the transport of ions across the cell membrane. Important Energy is required for both systems to work, wich is derived from root respiration. respiration. Factors influencing crop nutrition Internal factors Genetic factors Nutrition characteristics of species and varieties Morphological characteristics - shoot: root ratios - root development External faxtors Environmental factors - climatic and weather conditions - water supply - air (components) - light conditions (radicance) soil properties - Nutrient requirement, dynamics - Temperature requirement requirement - pH tolerance, salt tolerance - nutrient supply - soil atmosphere, moisture water: air ratio - soil pH, texture - soil organic matter - microorganisms pH TOLERANCE OF SEVERAL CROPS Acidic Optimum pH range is narrow Tolerant pH range is relatively wide SENSITIVE Rye Wheat Barley, Alfalfa Potato (sweet and white) Corn, Barley (several varieties) Sugarbeet, Sweetclover Tobacco, Cotton Peas Soybeans Beans Rice, Sunflower Cabbage Canola Lettuce Cucumber Strawberries Tomatoes Onions Buckwheat Lentil, Radish Carrots Source: Brady, 1990) Optimum pH ranges of different crops (Havlin et al. 2005) pH RANGES CROPS 4.5 Alfalfa Apples Barley Cabbage Corn Onions Peas Potatoes, Sweet Potatoes, White Sorghum Soybeans Wheat 5.0 5.5 6.0 6.5 7.0 7.5 RELATIVE YIELD OF CROPS AS AFFECTED BY SOIL pH (in percentages of maximum yield) pH CROP 4.7 5.0 5.7 6.8 7.5 Sweet clover 0 2 49 89 100 Barley 0 23 80 95 100 Alfalfa 2 9 42 100 100 Red clover 12 21 53 98 100 Corn 34 73 83 100 85 Soybean 65 79 80 100 93 Wheat 68 76 89 100 99 Oats 77 93 99 98 100 Source: Field Experiment Station, Ohio, USA 1983 RELATIVE SALT TOLERANCE OF CROPS Tolerant Moderately tolerant Moderately sensitive Sensitive Barley Barley, forage Alfalfa Apple Cotton Broccoli Broad bean Apricot Wheat grass, tall Sorghum Cabbage Bean Sudan grass Corn Carrot Bermuda grass Wheat Cowpea Celery Cucumber Grapefruit Lettuce Lemon Pea Onion Peanut Orange Rice, paddy Peach Soybean Sugarcane Potato Sugar beet Strawberry Tomato Brady N. (1990) Principal soil conditions resulting Mineral Stresses on Plants (Epstein & Bloom, 2005) Mineral Stress Characteristics Salinity High salt concentrations, mostly sodium (Na) Sodicity Excess (more than 10 %) percentage of Na in the cation exchange sites Heavy metal and Al toxicities Common in acid soils, due to the increased solubility of these metals Micronutrient deficiencies Common in calcareous soils, due to the low solubility level of these elements (insoluble, precipitated forms) Low Ca/Mg ratios Serpentine soils with Ca/Mg ratio 1:1 or lower (optimum is 2:1 to 3:1) Low soil fertility Low levels of available nutrients, mostly N and P Soil Conditions Resulting Shortages of Available Nutrients and Inducing Nutrient Deficiencies for Crop Plants Nutrient Soil Conditions resulting Shortages in availability - inducing deficiency N Excess leaching with heavy rainfall low organic matter content of soils, burning the crop residue P Acidic, organic, leached, and calcareous soils, high rate of liming K Sandy, organic, leached, and eroded soils, high liming application, intensive cropping system Ca Acidic, alkali, or sodic soils Mg Similar to calcium S Low organic matter content of soils, use of N and P fertilizers containing no sulfur, burning the crop residue Fe Calcareous soils, soils high in P, Mn, Cu or Zn, high rate of liming Zn Highly leached acidic soils, calcareous soils, high levels of Ca, mg, and P in the soils Mn Calcareous silt and clay, high organic matter, calcareous soils B Sandy soils, naturally acidic leached soils, alkaline soils with free lime Mo Highly podzolized soils, well-drained calcareous soils Source: Fageria et al., 1991. AVAILABILITY RANGES OF NUTRIENT ELEMENTS DEPENDING ON SOIL pH Maximum availability for the majority of nutrients: at pH = 6.5 i.e. under slightly acidic conditions Availability of metal cations (mostly microelements) increases with acidity, with the exception of Molybdenum.