* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chronotropic Effects of Select Cardiovascular Drugs on the
Orphan drug wikipedia , lookup
Discovery and development of beta-blockers wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Drug design wikipedia , lookup
Theralizumab wikipedia , lookup
Drug discovery wikipedia , lookup
Neuropharmacology wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Plateau principle wikipedia , lookup
Psychopharmacology wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Pharmacognosy wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Prescription costs wikipedia , lookup
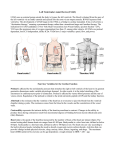
Chronotropic Effects of Select Cardiovascular Drugs on the Developing Vertebrate Heart 1 Jacqueline McLaughlin, 2Vinod Jeyaretnam 1 Penn State Lehigh Valley; 2Undergraduate student Abstract An experiment was performed to investigate the effects of two known cardiovascular drugs on the developing 4-chambered vertebrate heart using the embryonic chicken as the model system. Ninety percent of pregnant women take some type of drug: whether that is prescription, over-the-counter, or social in nature. The FDA has developed a grading rubric to rate a drug that may be used during pregnancy based on the risk that drug poses to the developing fetus. The human fetus is particularly vulnerable to birth defects between the third and eighth weeks of development – which directly correlates with the timing of major heart developmental stages. In the following study, two known cardiovascular drugs with opposing effects were exposed to isolated chicken hearts at times during development that denote crucial heart organogenic events in order to directly study chronotropic effects. Ro 40-5967 is a known potent T-type calcium channel antagonist, while NKH477 is known to have positive inotropic and chronotropic effects. The pharmacokinetics and pharmacodynamics of both drugs is fairly well understood in numerous adult vertebrate model systems; however, no information exists on the effects of these drugs on a vertebrate embryo. Due to known chronotropic effects in the adult model system it was hypothesized that Ro 40-5967 would decrease heart rate while NKH477 would increase it. In this study, the effects of Ro 40-5967 and NKH477 were tested on “isolated” embryonic chicken hearts using the live cell Bioptechs ® thermal control system, the Delta T-EDU. Baseline heart rates (HR) were determined for in vivo, in vitro whole embryo, and in vitro isolated hearts. Both drugs were administered at 10-4, 10-3, 10-2, and 10-1 mg/mL, and at each concentration the average HR was determined and arrhythmias noted. The proposed hypothesis was not supported. There was a clear difference between 6-day and 7-day embryos resulting in a “D” and “C” FDA drug risk grade for Ro 40-5967 and NKH 477, respectively. In conclusion, it is recommended that neither drug be administered to a pregnant woman. Introduction Drugs administered to pregnant mothers have the potential to cross the placenta and reach the fetus. The chicken embryo is a classic organism used to investigate vertebrate heart development and the pre-natal toxicity of maternally administered drugs. The United States Food and Drug Administration (FDA) classifies drugs according to the degree of risk they pose for the fetus. How a drug affects Figure 1- EDUSTGE 7522-05-08 of the Delta T-EDU Bioptechs apparatus. 1 a fetus depends on the fetus’s stage of development and the strength and dose of the drug. Certain drugs taken early during pregnancy may or may not affect the fetus. However, the fetus is particularly vulnerable to birth defects between the 3rd and the 8th week after fertilization, when its major organs are developing. The vertebrate 4-chambered heart, along with the eyes and brain, is among the first organs to develop in humans and chickens. A one-month human heart resembles that of a 2.5 – 3- day chick heart; a twomonth human heart resembles that of a 6-day chick embryo. Herein, 6-day and 7-day chick embryos were tested to simulate a developing human heart system during known, critical heart development events. Figure 2. Comparative embryology of the 60-hour chicken model system (left) and a one-month-old human (right). 6-day and 7-day Results According to previous research conducted on adult vertebrate model systems such as hamsters, rats, dogs, and humans, Ro 40-5967 has been shown to decrease HR linearly as concentration increases. This is clearly evident in the 7-day heart, but not the 6-day heart suggesting that the embryonic pacemaker, the sinus venosus, is still controlling heart rate at 6-days and reacts differently than the mature sinoatrial node at 7-days to this drug. 2 Ro 40-5967 6-day Average Heart rate (bpm) 170 150 130 y = -20.833x2 - 121.77x - 32 110 90 70 50 -6 -4 -2 0 Log of Drug Concentration (mg/mL) Ro 40-5967 CMRL 1066 1X Poly. (Ro 405967) Figure 3. A scatter plot depicting the heart rate of 6-day “isolated” chicken hearts. CMRL 1066 1X was used as the control. An increase in heart rate (bpm) was observed at the three lowest concentrations of Ro 40-5967. The highest concentration saw a dramatic drop in bpm. Atrial flutter was also observed. Average Heart Rate (bpm) Ro 40-5967 7-Day y = -8.4x + 58.833 130 120 110 100 90 80 70 60 50 -6 -4 -2 0 Log of Drug Concentration (mg/mL) Ro 40-5967 CMRL 1066 1X Linear (Ro 405967) Figure 4. A scatter plot depicting the bpm of 7-day “isolated” chicken hearts. CMRL 1066 1X was used as the control. A linear decrease in bpm was observed. Atrial flutter was observed at the highest concentration of Ro 40-5967. 3 NKH477 6-Day 160 Average Heart Rate (bpm) 150 140 130 y = -13.792x2 - 84.842x + 21.125 120 110 100 90 NKH477 CMRL 1066 1X Poly. (NKH477) 80 -6 -4 -2 0 Log of Drug Concentration (mg/mL) Figure 5. A scatter plot depicting the bpm of 6-day “isolated” chicken hearts. CMRL 1066 1X was used as the control. An increase in bpm was observed over the three lowest concentrations. The highest concentration saw a slight drop in bpm. Average Heart Rate (bpm) NKH477 7-Day 150 145 140 135 y = -2.75x2 - 6.45x + 143.58 130 125 120 115 NKH477 CMRL 1066 1X Poly. (NKH477) 110 -4.5 -2.5 -0.5 Log of Drug Concentration (mg/mL) Figure 6. A scatter plot depicting the average heart rate of day seven “isolated” chicken hearts. CMRL 1066 1X was used as the control. An increase in heart rate was observed. The highest concentration saw a slight drop in bpm. Conclusion Overall, these data suggest that Ro 40-5967 and NKH477 have opposing effects on 4 embryonic vertebrate heart rate, and neither should be used in a clinical setting until proper dosage and deleterious effects are further analyzed. Data also strongly suggest that the affect of these drugs on the developing heart depends on the embryo’s stage of development and the strength and dose of the drug. Based on the data collected and the rubric for grading FDA drug risk during pregnancy, the following ratings for each drug are suggested: • Ro 40-5967 gets a “D” because studies in humans, or investigational or post marketing data, have demonstrated fetal risk. Nevertheless, potential benefits from the use of the drug may outweigh the potential risk. • NKH477 gets a “C” because risk can not be ruled out. Adequate, wellcontrolled human studies are lacking, and animal studies have shown a risk to the fetus or are lacking as well. There is a chance of fetal harm if the drug is administered during pregnancy; but the potential benefits may outweigh the potential risks. Future experimentation will be conducted to further understand when, exactly, the intrinsic conduction system of the chicken heart is fully developed, and how these and other cardiovascular drugs directly effect the Ca+2 based, T-type channels that regulate the “pace-maker” potential. References Adenylate cyclase (2012, February 5). In Wikipedia. Retrieved April 1, 2012 cAMP-dependent pathway (2012, February 5). In Wikipedia. Retrieved April 1, 2012 Catterall, W. (2000). Structure and regulation of voltage-gated ca 2 channels. Annual Review of Cell and Developmental Biology, 16, 521-555. Clozel, J., Ertel, E., & Ertel, S. (1997). Discovery and main pharmacological properties of mibefradil (ro 40-5967), the first selective t-type calcium channel blocker. Journal of Hypertension, 15(5), S17-S25. Hayashida, N., Chihara, S., Tayama, E., Takaseya, T., Enomoto, N., Kawara, T., & Aoyagi, S. (2001). Antiinflammatory effects of colforsin daropate hydrochloride, a novel water-soluble forskolin derivative. Annals of Thoracic Surgery, 71, 19311938. Retrieved March 31, 2012, from Elsevier Science. Hussar, D. A. (1998, July). New Drugs 98, part IV: Mibefradil dihydrochloride [Electronic version]. Nursing and Allied Health Source, 28(7), 36-37. Kikura, M., Morita, K., & Sato, S. (2004). Pharmacokinetics and a simulation model of colforsin daropate, new forskolin derivative inotropic vasodilator, in patients undergoing coronary artery bypass grafting. Pharmacological Research, 49, 275281. Retrieved March 31, 2012, from Elsevier Science. 5 McLaughlin, J. S., & McCain, E. R. (1998). Developmental and physiological aspects of the chicken embryonic heart. Tested Studies for Laboratory Teaching, Volume 20. (C.A. Goldman, editor). Proceedings of the 20th Workshop/Conference of the Association for Biology Laboratory Education (ABLE), 20: 85-100 Posicor package insert (Roche-US). New 6/97, Rec 8/97; Rev 12/97, Rec 2/98 Ray, D., & Dyson, D. (1995). Calcium channel blockers. Clinical Obstetrics and Gynecology, 38(4), 713-721. Yoshida, H., Tanonaka, K., Miyamoto, Y., Abe, T., Takahashi, M., Anand-Srivastava, M. B., & Takeo, S. (2001). Characterization of cardiac myocyte and tissue β-adrenergic signal transduction in rats with heart failure. Cardiovascular Research, 50, 34-45. Retrieved March 31, 2012, from Elsevier Science. 6