* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download History of anticoagulant therapy
Survey
Document related concepts
Pharmacognosy wikipedia , lookup
Drug design wikipedia , lookup
Neuropharmacology wikipedia , lookup
Drug interaction wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Drug discovery wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Prescription costs wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Theralizumab wikipedia , lookup
Discovery and development of direct Xa inhibitors wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Dydrogesterone wikipedia , lookup
Discovery and development of direct thrombin inhibitors wikipedia , lookup
Transcript
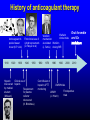
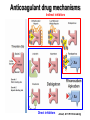
Update on the New Oral Anticoagulants Eliot Williams, MD PhD Division of Hematology & Medical Oncology Nothing to disclose History of anticoagulant therapy Anticoagulant in spoiled sweet clover (K.P. Link) 1910 1920 1930 First clinical use of 4-hydroxycoumarin (O. Meyer et al) 1940 1950 Heparin Clinical use of discovered heparin by medical Requirement student for plasma (McLean) cofactor discovered (K. Brinkhous) 1960 Warfarin Warfarin clinical trials mechanism elucidated Warfarin (J. Suttie) dosing/INR 1970 Cont infusion of heparin; aPTT monitoring 1980 1990 2000 Oral thrombin and Xa 2010 LMWH trials LMWH (J. Hirsch) Fondaparinux trials New oral anticoagulants • Dabigatran (Pradaxa®) – thrombin inhibitor – FDA approval 2010: stroke prevention in nonvalvular Afib • Rivaroxaban (Xarelto®) – Xa inhibitor – FDA approval 2010/11: postop VTE prophylaxis, stroke prevention in Afib, treatment of VTE • Apixaban (Eliquis®) – Xa inhibitor – FDA approval 2012: stroke prevention in Afib; approved 2014 for VTE prophylaxis after major orthopedic surgery • Edoxaban – Xa inhibitor – Not yet FDA approved Anticoagulant drug mechanisms Indirect inhibitors Direct inhibitors Ansell, 2011 HTRS meeting Edoxaban Pharmacology of oral anticoagulant drugs Warfarin New agents Bioavailability 99% 6-80% (some active drug in large bowel) Tmax 72-96 hours 2-4 hours Half-life 40 hours 5-17 hours Metabolism Cytochrome P450 Biliary/Renal Drug Interactions Many Not so many Food Interactions Yes No Genetic Variation Major effects Minor effects (?) Monitoring PT/INR None Reversal Vit K/PCC/FFP PCC? Dialysis? Cost per month of oral anticoagulants • Rivaroxaban (20 mg/day) : $290 • Dabigatran (150 mg bid): $290 • Apixaban (5 mg bid): $147 • Warfarin (7.5 mg/day): $31 Source: UWHC Pharmacy Dabigatran • Dose – Stroke prevention in A fib: 110-150 mg bid • 110 mg dose not available in US • For patients with CrCl 15-30: 75 mg bid • Not recommended for CrCl < 15 or dialysis dependent – Postop VTE prophylaxis*: 150-220 mg once daily – Prevention of recurrent VTE*: 150 mg bid • Less than 10% absorbed; relatively high rate of GI side effects • Crosses the placenta – do not use during pregnancy • Drug may degrade over time after exposure to air – must be kept in original packaging Unused tablets should be discarded after 90 days * Not FDA-approved indication Rivaroxaban • Dose: – Stroke prevention in Afib: 15-20 mg once daily – Post op VTE prophylaxis: 10 mg once daily – Acute VTE treatment: 15 mg twice daily – Secondary prevention of VTE: 20 mg once daily – Acute coronary syndrome*: 2.5-5 mg twice daily • Use with caution in moderate renal impairment (CrCL 30-49); 15 mg/day dose recommended – Avoid use if CrCl < 30 (not dialyzable) • Avoid use in severe liver disease *Not FDA-approved indication Apixaban • Dose: – Stroke prevention in Afib: 5 mg bid • 2.5 mg bid if age >80, weight < 60 kg, or serum creatinine > 1.5 – Post op VTE prophylaxis*: 2.5 mg bid – Secondary prevention of VTE*: 2.5-5 mg bid – Treatment of acute VTE*: 10 mg bid – Secondary prevention of VTE*: 5 mg bid • Avoid use in severe liver disease (75% biliary excretion) *Not FDA-approved indication NOACS for ATRIAL FIBRILLATION NEW ORAL ANTICOAGULANTS VS WARFARIN IN NON-VALVULAR ATRIAL FIBRILLATION Trial RE-LY Drug being compared Dabigatran # subjects CHADS2 (mean) TTR (median) 18,113 2.1 67% (two doses) ROCKET-AF Rivaroxaban 14,264 3.5 58% ARISTOTLE Apixaban 18,201 2.1 66% ENGAGE AF-TIMI 48 Edoxaban (two doses) 21,105 2.8 68% • • • • All randomized; RE-LY unblinded All designed as non-inferiority trials Primary outcome was stroke or embolism All funded by drug manufacturer NEJM 2009; 361: 1139 NEJM 2011; 365:883 NEJM 2011; 365:981 NEW ORAL ANTICOAGULANTS VS WARFARIN: RISK OF STROKE OR EMBOLISM Dabigatran 150 mg bid Rivaroxaban 20 mg qd Apixaban 5 mg bid Edoxaban 60 mg qd Combined Ruff et al, Lancet 2013 NEW ORAL ANTICOAGULANTS VS WARFARIN: SECONDARY EFFICACY AND SAFETY OUTCOMES Ruff et al, Lancet 2013 NEW ORAL ANTICOAGULANTS VS WARFARIN: RISK OF MAJOR BLEEDING Dabigatran 150 mg bid Rivaroxaban 20 mg qd Apixaban 5 mg bid Edoxaban 60 mg qd Combined Ruff et al, Lancet 2013 Bleeding rates with dabigatran vs warfarin as a function of age D 110 D 150 Warfarin Warfarin D 150 D 110 • Intracranial bleeding lower with dabigatran at all ages • Extracranial bleeding rates higher with dabigatran above age 75 Circulation 2011;123:2363 Dabigatran use associated with higher risk of coronary events ←Risk lower with dabigatran Risk higher with dabigatran→ Arch Intern Med 2012;172:397 LESSONS FROM AF TRIALS WITH NEW ORAL AGENTS • Main result: New agents at least as effective as warfarin, can be given without routine monitoring • Other/unexpected findings: – Reduction in intracranial bleeding – Higher MI rates (dabigatran) – Higher rates of GI bleeding (active drug in lower intestine) – Extracranial bleeding risk higher in older patients Relative efficacy and safety of apixaban vs warfarin, according to adequacy of individual INR control The benefit of switching from warfarin to a NOAC appears to be greatest in patients with relatively poor INR control Favors apixaban Favors warfarin Wallentin et al, Circulation 2013 Can the new oral agents be used in patients with mechanical valves? • Randomized trial of dabigatran vs warfarin in patients with mechanical valves showed more DO NOT USE NOACs IN PATIENTS WITH thrombotic complications (5% vs 0) and more MECHANICAL bleeding (4% vs 2%) with VALVES dabigatran (Eikelboom et al, NEJM 2013; 369:1206) NOACS for TREATMENT OF VTE Efficacy of NOACs for treatment of acute VTE is comparable to warfarin meta-analysis of phase 3 trials J Thromb Haemost 2014;12:320 Safety of NOACs for treatment of acute VTE is superior to warfarin meta-analysis of phase 3 trials J Thromb Haemost 2014;12:320 NOACs for treatment of VTE • Efficacy comparable to warfarin • Modest safety advantage • Practical advantages – No monitoring – No injections – No transitioning – single agent treatment – Shorter hospital stay NOACS for VTE PROPHYLAXIS NOACs vs LMWH after total hip or knee arthroplasty A systematic review of the literature Dabigatran vs LMWH Xa inhibitors vs LMWH Mortality Less thrombosis, Symptomatic more bleeding with NOACs DVT Nonfatal PE Major bleeding Ann Intern Med 2013;159:275 NOACS for ACUTE CORONARY SYNDROME New oral anticoagulants plus antiplatelet therapy in ACS: meta-analysis Favors NOA Favors placebo Non-significant decrease in overall mortality, large increase in risk for major bleeding Arch Intern Med 2012; 172:1537 Monitoring Effects of NOACs on routine coag tests • PT/INR and PTT are relatively insensitive to the effects of these drugs – Reagent-dependent – results will vary among labs • Normal PT and PTT do not rule out significant blood level of NOAC • If PT or PTT elevated → assume significant blood levels of NOAC • Thrombin time very sensitive to dabigatran effect – normal TT implies no drug on board – Rivaroxaban & apixaban do not affect TT Measuring blood levels of NOACs • Dabigatran: – Modified thrombin time assay (Hemoclot®) • Rivaroxaban and apixaban: – Anti-Xa activity (similar to LMWH assay) • Neither assay FDA-approved or widely available now • When to consider measuring drug level: – Detect/quantify overdose – Screen for drug accumulation (eg, impaired renal or liver function) – Assure low drug level prior to surgery Limited usefulness for assessing compliance due to short drug half-lives REVERSAL? • No specific antidote for any of the new OACs – New agents in development may solve this problem • Activated charcoal will reduce drug absorption if administered within a few hours of ingestion • Rivaroxaban & apixaban effect may be reversed by giving prothrombin complex concentrate (PCC) (limited data) • Dabigatran is dialyzable – 60% removed/3 hours, with rebound effect • Case reports suggest that recombinant factor VIIa (NovoSeven™) is ineffective vs dabigatran (Thromb Haemost 2012;108:585) Risk-benefit profile of NOACs vs warfarin remains favorable in patients with moderate renal insufficiency (GFR < 50) Meta-analysis of 9 phase III trials Thrombotic events Major bleeding VKA better VKA better NOAC better NOAC better % of drug excreted by kidneys → J Thromb Haemost 2014;12:337 Transitioning Transitioning to NOACs • Unfractionated heparin to NOAC: – Start NOAC when UFH infusion stopped • LMWH to NOAC: – Start NOAC 2 h before next scheduled sq dose of LMWH • Warfarin to NOAC: – When INR < 2.0 Transitioning from NOACs • NOAC to parenteral anticoagulant: – CrCl >30: start 12 hours after last NOAC dose – CrCl <30: start 24 hours after last NOAC dose • NOAC to warfarin: – CrCl >50: start warfarin 3 days before NOAC stopped – CrCl 31-50: start warfarin 2 days before NOAC stopped – CrCl 15-30: start warfarin 1 day before NOAC stopped Remember that NOACs can prolong PT/INR When to stop drug before surgery • Stop NOAC at least 3 drug half-lives prior to surgery – Dabigatran: 42-51 h – Rivaroxaban: 15-27 h – Apixaban: 24-48 h • Allow more time if: – Age > 75 – Impaired renal or liver function – High bleeding risk Who are the best candidates for new oral anticoagulants? • Patients who have unstable INR on warfarin not due to poor compliance • Reasonably good renal & hepatic function • No mechanical valve • Not pregnant (drugs cross placenta) • < 75 years old • No history of lower GI bleeding • Not at high risk for ACS (dabigatran)