* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 10 Powerpoint
Discovery and development of non-nucleoside reverse-transcriptase inhibitors wikipedia , lookup
Compounding wikipedia , lookup
Orphan drug wikipedia , lookup
Discovery and development of proton pump inhibitors wikipedia , lookup
Discovery and development of cyclooxygenase 2 inhibitors wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Drug design wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Neuropharmacology wikipedia , lookup
Prescription costs wikipedia , lookup
Pharmacognosy wikipedia , lookup
Drug interaction wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
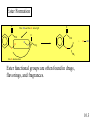
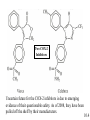
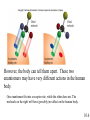
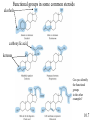
Chapter 10 Manipulating Molecules and Designing Drugs Is our society becoming dependent on prescription drugs? How harmful are illicit drugs? Herbal remedies: Is “natural” safer than synthetic? How are effective drugs designed? A wonder drug? The classic white willow tree, Salix alba, provides more than just shade and shelter for nature’s animals. Salicin is the key ingredient that is isolated from the tree and converted into one of our most reliable and heavily used drugs, aspirin! It is used as an antipyretic-fever reduction. White willow tree It is used as an analgesic-pain reliever, and as an anti-inflammatory agent. salicin 10.1 Semi-synthesis of aspirin: from natural product to marketed drug Salicylic acid White willow tree acetic acid anhydride Possible side effects: 1. Heartburn 2. Nausea 3. Reye’s Syndrom 4. Ringing in ears 5. Rash, itch 6. Shortness of breath acetylsalicylic acid (aspirin) acetic acid Would this drug make it through FDA scrutiny today? 10.1 Organic Chemistry – The Study of Carbon Compounds There are over 12 million known organic compounds. Why carbon? Carbon has the remarkable ability to bond in multiple ways: 10.2 Some different ways to represent molecular structures: CH3CH2CH2CH3 Ball-and-stick formula Structural formula Space-filling model CH3CH(CH3)CH3 n-butane and isobutane are isomers, molecules that have the same chemical formula, but with different structures and properties 10.2 Representing molecular structures with line-angle drawings: H H H C H H H H C C C C H C H = H H H H H iso hexane, or 2-methyl pentane H H H H C H H C C Each vertex (or end of a line) represents a carbon with an appropriate number of hydrogen atoms. C H C = H H H cyclopentane 10.2 10.2 Representations of benzene (C6H6), an important part of many drug molecules. 10.2 Functional groups - arrangements of groups of atoms which impart characteristic physical and chemical properties. 10.3 More Important Functional groups The presence and orientation of functional groups are responsible for the action of all drugs. See Chapter 9 for more info on functional groups. 10.3 Ester Formation O This OH and this H form H2O C O C O OH OH H H O C O CH 3 O O + H OH C CH 3 this O attaches here Ester functional groups are often found in drugs, flavorings, and fragrances. 10.3 What are the functional groups in the aspirin molecule? 1. Benzene ring 2. Carboxylic acid 3. Ester 10.3 Examples of polar molecules Functional groups may impart a polar aspect to a molecule. A polar molecule has partial charge separation. d- = “partial negative” d+ = “partial positive” 10.3 Salt Formation OH CH3 CH3 OH CH3 CH3 CH CH NH Pseudoephedrine freebase + H Cl CH CH NH2Cl Pseudoephedrine hydrochloride The salt forms are often preferred as drugs because of: • Increased water solubility • No odor • Higher melting points (longer shelf life) 10.3 Hormones, our chemical messengers, are produced by the body’s endocrine glands. Hormones encompass a wide range of functions and a similarly wide range of chemical composition and structure. Enzymes are proteins that act as biochemical catalysts, influencing the rates of chemical reactions. Aspirin works by inhibiting the production of certain enzymes responsible for producing specific hormones. 10.4 Aspirin and other drugs that are physiologically active, but not antiinfectious agents, are almost always involved in altering the chemical communication system of the body. Aspirin, as well as other non-steroidal antiinflammatory (NSAID) drugs, work by inhibiting the catalytic activity of certain enzymes. The mode of action is due to the ability of aspirin to block the action of cyclooxygenase (COX-1 and COX-2) enzymes from producing the hormonal compounds called prostaglandins. Certain prostaglandins are responsible for the body’s response to pain, inflammation, and fever. 10.4 Prostaglandins cause a variety of effects. They produce fever and swelling, increase sensitivity of pain receptors, inhibit blood vessel dilation, regulate the production of acid and mucus in the stomach, and assist kidney functions. By preventing COX-2 enzymes from catalyzing prostaglandin production, aspirin reduces fever and swelling. It also suppresses pain receptors and so functions as a painkiller. But the drug (as do other NSAIDs) also inhibit COX-1 enzymes that primarily make hormones that maintain proper kidney function and keep the stomach lining intact. Thus, NSAIDs drugs are not sufficiently selective to affect COX2 without shutting down COX-1 as well. 10.4 Researchers raced to create nearly a dozen new candidate drugs that block COX-2 alone. This work resulted in the emergence of a new class of medicines in the late 1990s called COX-2 inhibitors. Two wildly popular and heavily prescribed COX-2 inhibitors are Vioxx and Celebrex. These new “superaspirins” were touted as being safer and more effective than currently available NSAIDs. The premise is that since they do not act on the COX-1 enzymes, there should be fewer gastrointestinal (GI) side effects. The reality is that they have not been proven to be more effective or safer than aspirin or ibuprofen. In fact, they have been proven to be more dangerous than helpful. Use the Web to write a short essay on the life and death of “super aspirins.” 10.4 Two COX-2 Inhibitors Uncertain future for the COX-2 inhibitors is due to emerging evidence of their questionable safety. As of 2008, they have been pulled off the shelf by their manufacturers. 10.4 Aspirin, revisited – mode of action COX enzymes Prostaglandin production COX -1: liver function, stomach lining COX-2: fever, pain sensation, inflammation 10.4 Drug Discovery-serendipity Sir Alexander Fleming, British bacteriologist Working with Staphylococcus-a bacteria. A colleague working in same building was working with Penicillium notatum-a fungus that produces penicillin. Actual photo of petri dish Flemming showing inhibition of growth of bacteria where penicillin landed on the dish. Series of chance occurrences, and penicillin was discovered. 10.5 Drug Discovery-serendipity H O S N PhOCH2 N O Penicillin V “In the fields of observation, chance favors only the prepared mind” -Louis Pasteur CH3 CH3 COOH How many functional groups can you identify in the structure above? 10.5 Drugs may be broadly classified as: aspirin 1. Those that cause a physiological response in the body. anticancer drugs morphine 2. Those that kill foreign invading organisms. antibiotics antifungal agents 10.5 Drug interactions at the cellular level The lock and key model. A substrate is a substance whose reactions are catalyzed by an enzyme. Here you see a drug occupying the receptor site of the natural substrate. 10.5 The functional groups and their placement in three-dimensional space determines to a large degree a molecule’s biological activity. The portion of a molecule that determines the biological effects of a drug is called the pharmacophore. 10.5 Combinatorial Chemistry Combinatorial chemistry is the systematic creation of large numbers of small molecules in “libraries” that can be rapidly screened in vitro for potential new drugs. The benefits of using combinatorial chemistry: 1. Many molecules can be created at a rapid rate. 2. The cost of the procedure is much cheaper than traditional molecule synthesis. 3. Large libraries of bioactive lead compounds can be produced relatively inexpensively. 10.5 Chiral molecules have 4 different groups attached to a central atom. A chiral molecule and its nonsuperimposable mirror image are a special kind of isomer called enantiomers. Enantiomers have identical physical properties. The only way we can tell them apart is by seeing their effect on plane polarized light. 10.6 However, the body can tell them apart. These two enantiomers may have very different actions in the human body. One enantiomer fits into a receptor site, while the other does not. The molecule on the right will have (possibly) no affect on the human body. 10.6 Consider the two enantiomers above. Dextromethorphan is a safe cough suppressant. Levomethorphan is an addictive opiate. 10.6 Steroids perform many functions in the body: C A D This is the basic carbon skeleton for all steroids. B 10.7 Functional groups in some common steroids alcohols carboxylic acid ketones Can you identify the functional groups in the other examples? 10.7 Prescription, Generic, and Over-the-Counter Medicines A generic drug is chemically equivalent to the pioneer drug, but cannot be marketed until the patent protection on the pioneer drug has run out after 20 years. The lower priced drug is commonly marketed under its generic name, in this case alprazolam instead of Xanax. 10.8 St. John’s Wort 10.9 Ephedra – used safely for thousands of years as herbal remedy known as Ma Huang. In 2003, several deaths related to the ingredients led the FDA (in 2004) to ban ephedra products. At this time, there are ongoing discussions to make the product available again. 10.9 Ephedra The herb contains the amphetamine-like alkaloids – ephedrine and the less active pseudoephedrine. Pseudoephedrine is used as a decongestant Ephedrine is a bronchodilator (opens the airways), and a stimulant 10.9 Drugs of Abuse 10.10 Marijuana – Cannabis sativa THC, or delta-9-tetrahydrocannabinol is the active drug in marijuana. Medical uses continue to be argued, the Federal Government is opposed. 10.10 OxyContin contains oxycodone Oxycodone has been used safely for many years. But this formulation includes a time-released mechanism for long-lasting treatment of chronic pain. Drug abusers quickly determined how to get around the time-release and were able to rapidly ingest large amounts of oxycodone. The effects were said to be similar to heroin. Over-prescribed in the Appalachian areas in late 1990s and early 2000s. Many became addicted. 10.10