* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Amines and Amides
Survey
Document related concepts
Transcript
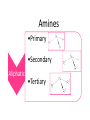
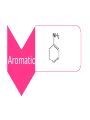
Amines and Amides Amine Amines are compounds that can be viewed as derivatives of ammonia. Characterized by Nitrogen joined to at least one alkyl group. Amines •Primary •Secondary Aliphatic •Tertiary Aromatic Amides If a carbonyl group lies between the nitrogen and R the compound is called an amide. They can be classified same as amine to aliphatic and aromatic, and aliphatic amides can be classified to Primary, Secondary and Tertiary. O H R N R' Physical Properties Primary and secondary amines have H atom attached to the N, therefore they are capable of intermolecular H-bonding. These forces are not as strong as those between alcohol molecules which have H bonded to O, a more electronegative element than N. This means that amines boils at lower temperature than alcohols. Tertiary amines have no H bonded to N and therefore can not form intermolecular H-Bonds All three classes of amines can H-bond with water. Amines with low molecular weight are soluble in water. Chemical Properties 1. Acetyle Chloride test - General test for amines - Evolution of heat and hydrogen chloride gas indicates a positive reaction(white Fumes). O CH3CH2NH2 + CH3CCl O CH3CH2NCCH3 + HCl 2. Rimini’s test - To distinguish Primary amine from secondary and tertiary - Positive result red coloration 3. Simon’s test - To distinguish secondary amine from primary and tertiary - Positive result Blue – green coloration 5. Bromine test - To distinguish aromatic from aliphatic amines - Positive test white ppt.