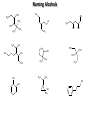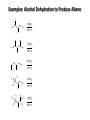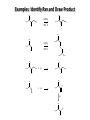* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Ether - Clayton State University
Aromaticity wikipedia , lookup
George S. Hammond wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Kinetic resolution wikipedia , lookup
Elias James Corey wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Hydroformylation wikipedia , lookup
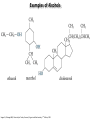
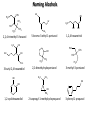
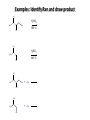
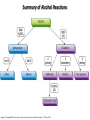
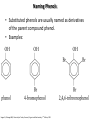
Chem 1152: Ch. 13 Alcohols, Phenols and Ethers Introduction • Alcohol: Any cmpd with a hydroxy (-OH) functional group attached on aliphatic carbon. R OH • Phenol: Hydroxy functional group attached to benzene ring, where the parent is a combination of the benzene ring and the (OH) group. OH • Ether: Oxygen with carbon attached on either side. O R Water structure analogs to alcohol and ether alcohol H ether OH O H R H OH O R R R Classification of Alcohols H Primary (1°) Hydroxy bearing C is attached to either 0 or 1 other C R Hydroxy bearing C is attached to 2 other C R Tertiary (3°) Hydroxy bearing C is attached to 3 other C O H R Secondary (2°) H O H R R H R O H H OH H H Examples of Alcohols Seager SL, Slabaugh MR, Chemistry for Today: General, Organic and Biochemistry, 7 th Edition, 2011 Examples of Alcohols • Antifreezes 1,2-ethanediol (ethylene glycol) • 1,2-propanediol (propylene glycol) Seager SL, Slabaugh MR, Chemistry for Today: General, Organic and Biochemistry, 7 th Edition, 2011 Rules for naming alcohols For single hydroxy (-OH) group • Step 1: Identify longest chain that includes (-OH) group. Drop –e from hydrocarbon name, and replace with ending –ol. • Step 2: Number this parent chain to give lowest number to carbon with attached (-OH) group. • Step 3: Locate position of (-OH) group. • Step 4: Locate and name all branches attached to parent chain. • Step 5: Include names of all branches (still in alphabetical order) in prefix of compound name. Include location of (-OH) group. • Note: Multiple (-OH) groups are named by addiing diol, triol, etc, to end of alkane without removing -e. 1 5 H3C 2 4 3 OH H3C 2 3 CH3 2-ethyl-1-pentanol HO 1 4 OH 2-methyl-1,4-butanediol Naming Alcohols HO CH3 H3C OH CH3 Br HO H3C H3C OH CH3 OH CH3 OH H3C H3C OH OH H3C CH3 HO CH3 H3C H3C OH CH3 H3C CH3 OH OH OH CH3 Naming Alcohols HO CH3 H3C OH CH3 Br HO H3C H3C OH CH3 2,2,4-trimethyl-3-hexanol OH CH3 5-bromo-3-ethyl-1-pentanol OH H3C H3C OH OH 1,2,4-hexanetriol H3C CH3 HO CH3 3-butyl-2,4-hexanediol H3C 2,2-dimethylcyclopentanol H3C OH CH3 H3C 3-methyl-3-pentanol CH3 OH OH OH CH3 1,2-cyclohexanediol 2-isopropyl-1-methylcyclopropanol 3-phenyl-1-propanol Physical Properties of alcohols- Solubility • Low MW alcohols (methanol, ethanol, propanol) are miscible with water. • As alkane chain gets longer, alcohol behaves more like an alkane: – More soluble in nonpolar organic solvents (benzene, CCl4, ether) Alcohols that look like water, behave like water. Alcohols that look more like alkanes behave like alkanes. Due to hydrogen bonding. Seager SL, Slabaugh MR, Chemistry for Today: General, Organic and Biochemistry, 7 th Edition, 2011 OH H3C H3C OH OH OH H H3C H3C CH3 Hydrogen bonding • Hydrogen bond: Attractive interaction of a hydrogen atom with an electronegative atom (e.g. N, O, F) from another molecule or chemical group. • The H must be covalently bonded to another electronegative atom. – This is why H on hydrocarbon chain does not participate in Hbonding. • H-bond relatively weak. • • Bond energy for C-H 413 kJ/mol H-bond energy 2 kJ/mol Seager SL, Slabaugh MR, Chemistry for Today: General, Organic and Biochemistry, 7 th Edition, 2011 Effects of hydrogen bonding • H-bonding between alcohol molecules increases BP for alcohol compared to similar MW alkane OH H3C OH H3C H3C CH3 O H3C Seager SL, Slabaugh MR, Chemistry for Today: General, Organic and Biochemistry, 7 th Edition, 2011 CH3 Alcohol Reactions 1. Alcohol Dehydration (Elimination Rxn): R R R R OH H H2SO4 R 180 C R R + H-OH R • Alcohol Hydration (Addition Rxn) R R R + H-OH R R R H2SO4 R R OH H Alcohol Dehydration to produce alkene • Alcohol Dehydration (Elimination Rxn): H H H C C H H H C C H H H H C C H HO H H C C H H2SO4 H H + H-OH H 2-butene 180 oC H H H C C H H C H C H + H-OH H 1-butene This rxn (at 180 °C) generates 2 products: 2-butene and 1-butene. The major product is 2-butene (90%) because both C=C bond carbons are attached to at least one other carbon. The minor product is 1-butene (10%) because only one of the C=C bond carbons is attached to at least one other carbon. The major product in these rxns will always be the one resulting in the highest number of carbon groups bonded to the C=C carbons. Alcohol dehydration in biochemistry H - O OC C H OH C CH2 - COO Enzyme - O OC citrate CH2 COO C - COO - H C + - COO cis-aconitrate This rxn is catalyzed by an enzyme rather than an acid in the human body. Alcohol dehydration rxns in general are involved in the formation of: Carbohydrates Fats Proteins H-OH Examples: Alcohol Dehydration to Produce Alkene CH3 H2SO4 H3C CH3 180 oC OH CH3 CH3 H2SO4 H3C CH3 180 oC OH H2SO4 CH3 CH3 H3C 180 oC OH CH3 H2SO4 CH3 H3C 180 oC OH H3C CH3 CH3 H3C CH3 CH3 OH H2SO4 180 oC Alcohol Dehydration to produce ether • Alcohol Dehydration (Elimination Rxn): OH H + H2SO4 O o R R alcohol R O R 140 C alcohol HO + H ether This rxn (at 140 °C) generates an ether and water. This rxn works mainly with primary alcohols. H Primary (1°) R Hydroxy bearing C is attached to either 0 or 1 other C H3C OH + H CH3 O H O H H2SO4 140 oC H3C OH H OH H H H3C O CH3 H2SO4 140 oC H3C O CH3 + + HO H HO H Alcohol Oxidation Oxidation: Loss of hydrogen or gain of oxygen. Oxidizing Agent: Compound that oxidizes another compound. KMnO4 (potassium permanganate) K2Cr2O7 (potassium dichromate) This rxn works different depending on whether alcohol is 1°, 2° or 3°. H-OH lost in oxidation rxns comes from the same carbon, rather than adjacent carbons as seen in dehydration rxns to form alkenes. H Primary (1°) Hydroxy bearing C is attached to either 0 or 1 other C R Hydroxy bearing C is attached to 2 other C R Tertiary (3°) Hydroxy bearing C is attached to 3 other C O H R Secondary (2°) H O H R R H R O H H OH H H Alcohol Oxidation for Primary Alcohol H H O C R O + H Primary alcohol (O) R C H + H-OH aldehyde O (O) R C OH Carboxylic acid Immediate product of oxidation of primary alcohol is aldehyde, which is then readily further oxidized to a carboxylic acid. The aldehyde product may be isolated before further oxidation by maintaining high temp. and boiling aldehyde out of rxn mixture. This is possible because aldehydes do not H-bond like alcohols and carboxylic acids. Alcohol Oxidation for Secondary and Tertiary Alcohols H R O C R O + (O) H Secondary alcohol R C R + H-OH ketone Product of oxidation of secondary alcohol is a ketone, which resists further oxidation. R R C R O H Tertiary alcohol + (O) NO RXN Examples: Identify Rxn and draw product CH3 H2SO4 H3C CH3 180 oC OH CH3 H2SO4 H3C 140 oC OH CH3 H3C CH3 + (O) + (O) OH CH3 H3C OH Examples: Identify Rxn and Draw Product CH3 CH3 H2SO4 H3C H3C CH3 CH3 o 180 C OH H CH3 CH3 H2SO4 H3C O H3C 140 oC OH H3C CH3 CH3 CH3 H3C CH3 + (O) H3C CH3 OH O CH3 CH3 H + H3C (O) H3C O OH (O) CH3 OH H3C O Summary of Alcohol Reactions Seager SL, Slabaugh MR, Chemistry for Today: General, Organic and Biochemistry, 7 th Edition, 2011 Phenols • Phenol: Hydroxy functional group attached to benzene ring, where the parent is a combination of the benzene ring and the (-OH) group. • Low MP solid that liquefies at room temp. with small amount of water. • Weakly acidic in water (due to conjugated pi bonds on benzene ring). – Can damage proteins in skin. • In dilute solutions, can be used as antiseptics and disinfectants. – Phenol first used by Joseph Lister in hospitals in 1800’s. – Phenol derivatives used today in Lysol, mouthwashes and throat lozenges. • Other phenol derivatives used as antioxidants. O OH + H2O - + H3O + Naming Phenols • Substituted phenols are usually named as derivatives of the parent compound phenol. • Examples: Seager SL, Slabaugh MR, Chemistry for Today: General, Organic and Biochemistry, 7 th Edition, 2011 Ethers • Ether: Oxygen with carbon attached on either side. • Naming ethers • Common Names 1. 2. Name the two groups attached to the oxygen then add the word ether If both groups the same, can be named with prefix di-. • IUPAC Names – O-R group is alkoxy. – The –yl ending of smaller R group is replaced by –oxy. O CH3 H3C butyl methyl ether 1-methoxybutane H3C H3C O p-methoxytoluene CH3 CH3 Cl CH3 H3C O dipropyl ether 1-propoxypropane CH3 isopropyl propyl ether 1-isopropoxypropane ethyl propyl ether 1-ethoxypropane H3C CH3 O CH3 CH3 O H3C CH3 O CH3 2-ethoxy-3,4dimethylhexane H3C O CH3 CH3 2-chloro-1-isopropoxypropane Properties of Ethers • Oxygen atom of ether can H-bond with water – Ethers more soluble in water than hydrocarbons, less soluble than alcohols of comparable MW. • Ethers cannot H-bond with other ethers in pure state – Results in low BP close to those of hydrocarbons of comparable MW. • Ethers mostly inert and unreactive – Why diethyl ether is useful solvent – Diethyl ether is also highly-flammable and was historically an important anesthetic – History Note: First physician to use diethyl ether as anesthesia was Crawford Long in 1842 Seager SL, Slabaugh MR, Chemistry for Today: General, Organic and Biochemistry, 7 th Edition, 2011 Thiols • • • • Thiols: Sulfur analogs of alcohols (-SH instead of –OH) Chemically- similar (i.e., form similar compounds) More volatile (lower BP) than alcohols but less water-soluble Thiols stink! – – – – This is how skunks defend themselves Chopped onions emit propanethiol Thiols found in garlic Ethanethiol added to natural gas (methane) so you can smell a leak • IUPAC Names for simple thiols – The –SH group is a sulfhydryl group. – Follow the same steps for naming as you do for alcohols, but do not modify alkane ending; instead add –thiol to end of parent. SH H3C H3C butanethiol SH SH CH3 2-butanethiol H3C CH3 CH3 2-methyl-3-hexanethiol Thiol/Sulfide Rxns Oxidation (Formation of disulfide bond) R H S + H R S + O O S R R S + H2O Reduction (breaking disulfide bond) S R R S + H H R H S + H R S Polyfunctional Compounds Substances that can contain more than 1 functional group. Ribose forms backbone of RNA (genetic transcription). Phosphorylated ribose becomes subunits of ATP, NADH, and several other compounds that are critical to metabolism. http://www.daviddarling.info/encyclopedia/R/ribose.html http://en.wikipedia.org/wiki/RNA Multistep Rxns The synthesis of most alcohols may require multiple steps (i.e., to get product X from reactant A, a product (B, C …X) must be created). To solve these problems, work backwards from the final product. O Oxidize 2° alcohol to form ketone. OH + O (O) + H-OH Use acid-catalyzed hydration (addition) to form alcohol. + H-OH H2SO4 OH The completed series of rxns. + H-OH H2SO4 OH + (O) O + H-OH