* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download INDUCTION OF SEVERE DISEASE IN HAMSTERS BY TWO
Dirofilaria immitis wikipedia , lookup
Onchocerciasis wikipedia , lookup
Brucellosis wikipedia , lookup
African trypanosomiasis wikipedia , lookup
Influenza A virus wikipedia , lookup
Trichinosis wikipedia , lookup
Yellow fever wikipedia , lookup
Oesophagostomum wikipedia , lookup
Sarcocystis wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
Rocky Mountain spotted fever wikipedia , lookup
Neonatal infection wikipedia , lookup
Ebola virus disease wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Middle East respiratory syndrome wikipedia , lookup
Orthohantavirus wikipedia , lookup
Schistosomiasis wikipedia , lookup
Antiviral drug wikipedia , lookup
West Nile fever wikipedia , lookup
Herpes simplex virus wikipedia , lookup
Hepatitis C wikipedia , lookup
Henipavirus wikipedia , lookup
Coccidioidomycosis wikipedia , lookup
Leptospirosis wikipedia , lookup
Marburg virus disease wikipedia , lookup
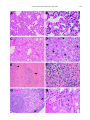
Am. J. Trop. Med. Hyg., 69(3), 2003, pp. 269–276 Copyright © 2003 by The American Society of Tropical Medicine and Hygiene INDUCTION OF SEVERE DISEASE IN HAMSTERS BY TWO SANDFLY FEVER GROUP VIRUSES, PUNTA TORO AND GABEK FOREST (PHLEBOVIRUS, BUNYAVIRIDAE), SIMILAR TO THAT CAUSED BY RIFT VALLEY FEVER VIRUS ANN F. FISHER, ROBERT B. TESH, JESSICA TONRY, HILDA GUZMAN, DONGYING LIU, AND SHU-YUAN XIAO Department of Pathology and Center for Biodefense and Emerging Infectious Diseases, and Department of Internal Medicine, University of Texas Medical Branch, Galveston, Texas Abstract. Adult golden hamsters inoculated subcutaneously with either of two sandfly fever group viruses, Punta Toro and Gabek Forest (Phlebovirus, Bunyaviridae), developed a fulminating fatal illness characterized by hepatic and splenic necrosis and interstitial pneumonitis. Most animals died within three days after infection; this was accompanied by high levels of viremia. Necropsy and histopathologic examination of the infected animals revealed pathologic changes involving multiple organs that resembled those described in Rift Valley fever. These two hamster-phlebovirus systems may serve as alternative animal models for Rift Valley fever and should be useful in studying the pathogenesis of severe phlebovirus infection and for testing potential therapeutic agents. febrile field worker in eastern Panama in 197213 and had been passaged four times in Vero cells; and 2) the prototype strain (Sud An 754-61) of GFV, which was originally isolated from a rodent (Acomys cahirinus) in Sudan in 1961.14 The latter virus had been previously passaged once in newborn mice and twice in Vero cell culture. Animals. Adult female Syrian golden hamsters (Mesocricetus auratus), 10−12 weeks of age, were used in all experiments. These were obtained from Harlan Sprague Dowley (Indianapolis, IN). The animals were cared for in accordance with the guidelines of the Committee on Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council) under an approved University of Texas Medical Branch animal use protocol. All work with the infected animals was carried out in BSL-3 facilities. Infection of animals and sample collection. Twenty-four hamsters were each inoculated with 104.6 plaque-forming units (PFV) of PTV, and 22 animals were each inoculated with 105.0 PFU of GFV. Both viruses were given by subcutaneous injection. Following inoculation of virus, four or more animals in each virus group were killed and sampled daily to follow the pathogenesis of the infections. Hamsters were anesthetized with Halothane (Halocarbon Laboratories, River Edge, NJ) and then exsanguinated by cardiac puncture. A portion of the blood was frozen for virus titration; the remainder was allowed to clot and the serum was used for liver enzyme studies. Immediately after death, the animals were dissected and their tissues were preserved in 10% buffered formalin for 24−48 hours, followed by 70% ethanol. Liver, spleen, kidney, adrenal gland, heart, lung, and a portion of small intestine were collected from all animals. From some of the hamsters, the entire gastrointestinal tract, brain, ovaries, pancreas, and lymph nodes were also collected. Histologic evaluation. After fixation, tissue samples were processed for routine embedding in paraffin. Four to 5-m−thick sections were made and stained by the hematoxylin and eosin method. Tissue sections were examined with an Olympus (Melville, NY) BX51 microscope equipped with UPlan/Apo objectives. For comparative purposes, a semi-quantitative analysis scheme, described previously15 was used with modifications. Liver sections were evaluated for inflammatory infiltration, hepatic necrosis, and steatosis, using the following scores: For INTRODUCTION Rift Valley fever (RVF) is a mosquito-borne epidemic disease of sub-Saharan Africa that affects cattle, sheep, goats, and humans.1–3 The disease is of considerable public health and veterinary importance in that region. The causative agent, RVF virus, is the type species of the genus Phlebovirus, family Bunyaviridae.4 Recent outbreaks of this disease in Egypt,3 Saudi Arabia, and Yemen5 indicate that RVF probably has the potential to invade other regions of the world where cattle, sheep, goats, and mosquitoes are abundant. For this reason, and because of the ease with which RVF virus can be aerosolized, there is now concern that it could be used as a bioterrorist agent.6,7 At present, there is no specific treatment or licensed human vaccine for RVF. Despite the need for a better understanding of the pathogenesis of RVF and for effective therapies for the disease, research with infectious RVF virus is inhibited by its current assignment to biosafety level-4 (BSL-4) status.8 In the United States, for example, work with RVF is restricted to a few government-sanctioned maximum containment (BSL-4) facilities. A more accessible model of the disease is needed. Rift Valley fever virus in lambs, calves, and kids produces a fulminant disease characterized by hemorrhage, focal liver necrosis, and death.1,2,9 In fact, the initial descriptions of RVF referred to the disease as “enzootic hepatitis.”9–11 Findlay11 was the first to report that hamsters were highly susceptible to RVF virus and that following infection these rodents developed a rapidly fatal disease similar to that seen in young sheep and goats. More recently, two other phleboviruses, Punta Toro virus (PTV) and Gabek Forest virus (GFV), have also been reported to produce severe disease in hamsters.12,13 In contrast to RVF virus, PTV and GFV are classified as BSL-2 and BSL-3 agents, respectively;8 consequently, PTV and GFV are available to more investigators. The objective of the present study was to characterize the pathology of PTV and GFV infections in hamsters and to evaluate the feasibility of using these two phleboviruses as models of the hemorrhagic fever form of RVF. MATERIALS AND METHODS Viruses. The two phleboviruses used were 1) the Adames strain of PTV, which was originally isolated from blood of a 269 270 FISHER AND OTHERS inflammation, 0 ⳱ none; 1 ⳱ occasional inflammatory cells (equivocal); 2 ⳱ mild but significant inflammatory infiltration; 3 ⳱ moderate inflammatory infiltration; 4 ⳱ marked or diffuse inflammatory infiltration; for necrosis, 0 ⳱ none; 1 ⳱ occasional apoptotic cells; 2 ⳱ mild necrapoptosis; 3 ⳱ moderate necrapoptosis; and 4 ⳱ focal or diffuse confluent necrosis. Steatosis was evaluated by a percentage portion of the parenchyma involved. Splenic tissue was evaluated for lymphoid reactive hyperplasia, lymphoid depletion/necrosis, and splenic macrophage proliferation (hyperplasia). These parameters were also scored from 0 to 4 (0 ⳱ normal; 1 ⳱ minimal; 2 ⳱ mild; 3 ⳱ moderate; and 4 ⳱ severe). In addition, lung tissue was evaluated for the degree of interstitial pneumonitis, with a degree of severity based on a 1 to 4 scale. Kidneys, ovaries, heart, adrenal glands, and pancreas were evaluated and described histologically. Immunohistochemical staining procedures were performed to detect the presence of viral antigens in the hamster tissue as previously described.15 After deparaffinization and dehydration, in xylene and decreasing concentrations of alcohol, formalin-fixed, paraffin-embedded tissue sections (3-4−m thick) were immersed in 3% hydrogen peroxide at room temperature for 30 minutes to block endogenous peroxidase activity. This was followed by an antigen retrieval step in a 10% target retrieval solution (Dako, Carpinteria, CA) for 30 minutes at 90°C. For detecting GFV antigens, the primary antibody used was mouse hyperimmune ascitic fluid (MIAF), prepared against the GFV prototype strain (Sudan 754-61); it was used at a dilution of 1:100. For detecting PTV antigens, a polyclonal MIAF prepared against the Adames strain of PTV was used at a dilution of 1:100. The incubation of the primary antibody was done overnight at 4°C. For subsequent detection of bound antibody, a mouse-on-mouse ISO-IHC kit (InnoGenex, San Ramon, CA) was used, following the manufacture’s instructions and as previously described.15 Virus assay. Blood from the hamsters infected with GFV was titrated by plaque assay in monolayer cultures of Vero cells. Serial 10-fold dilutions of each blood sample were prepared in phosphate-buffered saline (pH 7.4), containing 10% fetal bovine serum. Duplicate wells of 24-well microplate cultures of Vero cells were inoculated with each dilution. Cultures were inoculated at 37°C and plaques were counted 4−6 days later. Virus titers were defined as the number of PFU/mL of blood. The levels of viremia in hamsters infected with PTV were not determined because this information is available in another publication.13 Liver enzymes. Levels of alanine aminotransferase (ALT) were done on sera from the infected animals, using a commercial kit (Infinity ALT; Sigma Diagnostics, St. Louis, MO) according to the manufacturer’s instructions. TABLE 1 Serum levels of alanine amino transferase (ALT) in hamsters infected by Punta Toro virus (PTV) and Gabek Forest virus (GFV)* ALT levels (units/L) PTV-infected GFV-infected Day of infection n Mean SD n Mean SD 1 2 3 Negative control Positive control 4 5 5 42.1 70.7 427.9 26.5 353.6 8.6 25.0 189.4 4 5 7 40.3 293.4 328.3 12.8 115.3 46.1 142.6 121.5 * n ⳱ number of animals tested; Negative control ⳱ normal hamster serum; Positive control ⳱ serum from yellow fever virus–infected hamster. the mean ALT level was not significantly elevated; on day 2, it was slightly elevated for PTV-infected; and by day 3, it was markedly elevated, indicating hepatocellular damage and/or dysfunction. Pathologic findings in PTV-infected hamsters. In the first two days following infection, the organs appeared grossly normal at necropsy. However, by day 3, the small intestines appeared hemorrhagic (dark red) (Figure 1A); the contents were mahogany in color and liquid in consistency. Upon his- RESULTS Clinical and laboratory findings in PTV-infected hamsters. All of the animals appeared ill (extreme lethargy, anorexia, ruffled fur), and all but one were dead by the third day of infection. One animal was still alive on day 4 when the experiment was terminated. The ALT levels in the sera of infected hamsters during the first three days of infection are shown in Table 1. On day 1, FIGURE 1. A, Gross view of gastrointestinal organs from a hamster two days after infection with Punta Toro virus. The dark red discoloration of the small bowel corresponds to internal hemorrhages. B, Micrograph of the duodenum from the hamster showing hemorrhagic necrosis of the mucosal villi, with relative sparing of the bottom of the crypts (hematoxylin and eosin stained, magnification × 20). ANIMAL MODEL FOR PHLEBOVIRUS INFECTION tologic examination, abnormalities were found to be limited to the duodenum, which consisted of hemorrhage and necrosis of the mucosal villi (Figure 1B). The bottom of the mucosal crypts were spared. The pattern was similar to that seen in an acute ischemic process (infarction) of the bowel; the mechanism is unknown. The lower segments of the small bowel, colon, esophagus, and stomach were histologically normal. 271 In the liver, cytoplasmic condensation, formation of acidophil bodies, and nuclear fragmentation were observed in scattered individual hepatocytes on day 2 of infection (Figure 2A). By day 3, this had progressed to multifocal hepatocytic necrosis; however, the process was not as intense or severe as the necrosis seen in other hepatotropic viral diseases, such as yellow fever.15 There was no inflammatory cell infiltration, and no fatty changes were observed in the liver. Confluent FIGURE 2. Histologic changes in hamsters infected with Punta Toro virus. A, Liver, post-infection day (PID) 2. (magnification × 100). B, Spleen, PID 2, showing marked necrosis involving both the lymphoid zone and the red pulp (magnification × 50). C, Lung, PID 2, showing atelectasis (magnification × 50). D, Lung, PID 2, showing hemorrhagic necrosis (magnification × 100). E, Adrenal gland, PID 3, showing band-like cortical necrapoptosis. The arrowhead shows changes in superficial layer of the cortex (sub-capsular) (magnification × 25). F, Adrenal gland, PID 3, showing similar changes involving the corticomedullary junction (arrow) (magnification × 100). (Hematoxylin and eosin stained.) 272 FISHER AND OTHERS coagulation necrosis was not observed. The spleen exhibited marked necrosis involving both the lymphoid zone and the red pulp. The process was more focal in the lymphoid areas (Figure 2B). The lungs showed focal interstitial inflammation, and diffuse atelectasis was observed in a few hamsters (Figure 2C) on days 2 and 3. These changes were also seen in some hamsters on day 1. In addition, hemorrhagic pulmonary necrosis was seen in some hamsters on day 2 (Figure 2D). The adrenal glands had a peculiar pattern of necrosis, involving the cortex. Bands of cells became apoptotic in the most superficial layer of the cortex (sub-capsular) (Figure 2E) and at the corticomedullary junction (Figure 2F) corresponding to the zona glomerulosa and zona reticularis, respectively. The other regions and the medulla were normal. In addition, reactive hyperplasia accompanied by lymphoid necrosis was observed in the few lymph nodes examined, similar to changes in the spleen. No histologic abnormalities were found in the pancreas, kidneys, heart, or brain of the animals examined. Clinical and laboratory findings in GFV-infected hamsters. Gabek Forest Virus also produced a fulminant infection in the animals; all of the hamsters were either gravely ill or dead by the third day. The mean levels of viremia in this group of animals are shown in Figure 3. Within 24 hours, all of the hamsters had detectable viremia (mean ⳱ 102.8 PFU/ mL). On the second and third days of infection, the viremia with GFV was markedly elevated and sustained (means ⳱ 107.4 and 107.8 PFU/mL, respectively). The ALT levels in the GFV-infected hamsters paralleled the viremia. The ALT levels were slightly elevated on day 1, but were markedly elevated on days 2 and 3 (Table 1). Histopathologic findings in GFV-infected hamsters. On day 1 of infection, spleens from the four animals examined exhibited mild reactive lymphoid hyperplasia. The lungs from two of the four animals showed changes resembling severe interstitial pneumonitis (IP). However, tissue from the gastrointestinal tract, pancreas, kidneys, liver, adrenal glands, and heart were all normal histologically. On day 2, marked histologic changes were observed in many organs of five GFV-infected hamsters examined. Their spleens showed necrosis of both white and red pulps. Necrosis in the affected spleens was diffuse, with karyorrhexis noted in the red pulp. The liver changes ranged from patchy inflammatory infiltration with increased sinusoidal lymphocytes, Kupffer’s cells, and scattered necrapoptotic bodies, to severe necrosis. However, there was no steatosis. The lungs contin- FIGURE 3. Daily mean ± SD viremia in hamsters infected with Gabek Forest virus. PFU ⳱ plaque-forming units. ued to show interstitial pneumonitic changes (Figure 4A and B) in all animals, with severity scores ranging from 2 to 4. In addition, diffuse hemorrhages were seen in two of the hamsters. Two hamsters showed mild to moderate glomerular necrosis in the kidneys. No pathologic changes were seen in the renal tubules. Pancreatic necrosis was seen in only one hamster; it was characterized by scattered small foci of karyorrhexis in the acini. No pathologic changes were observed in the gastrointestinal tract or in the myocardium. Eight surviving hamsters were examined on day 3 of infection. Their spleens showed severe necrosis (score ⳱ 4+), with depletion of lymphoid tissue (Figure 4E and F). In some hamsters, reactive lymphoid hyperplasia co-existed with lymphoid depletion (different areas), a phenomenon also observed in a hamster model of severe yellow fever.15−17 In the liver, necrapoptosis varied in severity, ranging in score from 2 to 4. The hamsters with severe hepatic necrapoptosis (scores of 3 and 4) also showed mild steatosis (Figure 4D). The lungs exhibited variable histologic abnormalities. The severity of IP ranged from 1+ to 4+. The hamster with 4+ IP also had evidence of necrosis. Severe pulmonary edema was observed in one of the hamsters (Figure 4C). Pancreatic necrosis was not a consistent finding; it was present only in three hamsters and ranged from multifocal individual cell necrosis to focal acinar loss. Three hamsters had duodenal tissue available for assessment; two of the three animals demonstrated mild necrapoptosis of cells in the lamina propria. Other segments of the gastrointestinal tracts were normal. The adrenal gland from one animal examined showed mild scattered necrosis in the cortex. In addition, ovaries from two hamsters were examined; both showed necrosis of the stroma and follicles (eggs) (Figure 4G). Scattered cell necrosis was observed in glomeruli of all hamsters examined, with histologic scores ranging from 2 to 3 (Figure 4H). As in the first two days of infection, no abnormalities were observed in the myocardium on day 3. Detection of viral antigen in tissues of infected hamsters. Tissue sections were subjected to immunohistochemical staining to detect the presence of antigens of the infecting viruses, as described in the Materials and Methods. Tissues from uninfected hamsters or hamsters infected with yellow fever virus15 were processed similarly and used as negative controls. None of the control samples stained positively. In hamsters infected with PTV, the liver showed positive staining, ranging from hepatocytes around the central veins and the portal tracts (perivascular distribution of viral antigens), to scattered individual hepatocytes in other regions (usually with morphologic changes of cellular degeneration) (Figure 5A), to larger areas with overt necrosis (Figure 5B). Adrenal glands showed antigen positivity in the superficial cortical zone, and the cortical zone adjacent to the medulla, corresponding to zones with necrosis as seen in the hematoxylin and eosin−stained sections as described earlier in this report. Scattered macrophages in the interstitial areas of the parotid glands and lungs were also positive. In addition, renal tubular epithelia and myocardium of heart also showed weak focal positivity. Interestingly, no positive staining was observed in spleen or pancreas. In tissues from hamsters inoculated with GFV, immunohistochemical staining of the spleen, myocardium, and pancreas was negative for GFV antigen. In contrast, the tubular epithelia of kidneys were positive (in more than 60% of tubules) (Figure 5C). Positive immunohistochemical straining was also ANIMAL MODEL FOR PHLEBOVIRUS INFECTION 273 274 FISHER AND OTHERS ← FIGURE 4. Histopathology of hamsters infected with Gabek Forest virus. A, Lung, post-infection day (PID) 2, showing interstitial pneumonitis and focal consolidation, and thickening of the septa containing mononuclear inflammatory cell infiltration (magnification × 50). B, Lung , PID 2, showing interstitial pneumonitis and thickened septa with mononuclear inflammatory cells and a few neutrophils (magnification × 100). C, Lung, PID 3, showing diffuse edema and pink amorphous materials in the alveolar spaces (magnification × 50). D, Liver, PID 3, showing multifocal necrapoptosis (arrowheads) and cytoplasmic vacuolization (magnification × 100). E, Spleen, PID 3, showing marked lymphoid depletion due to necrosis. Only small residual foci of lymphoid areas are present (arrowheads) (magnification × 25). F, Spleen, PID 3, showing necrosis of lymphoid area and the red pulp. Note the loss of small lymphocytes, marked karyorrhexis (“nuclear dust”) and residual large immunoblasts (upper right corner) (magnification × 50). G, Necrosis of an ovary, PID 3. Note the cell damages in the stroma (arrowheads) and center of a follicle (magnification × 50). H, Scattered apoptosis involving a glomerulus in the kidney, PID 3. The tubules were normal (magnification × 100). (Hematoxylin and eosin stained.) seen in hepatocytes, especially in cells surrounding the central veins and the portal tracts. Scattered individual hepatocytes were also strongly positive. Only occasional macrophages in the septa of lungs showed a positive staining reaction (Figure 5D). DISCUSSION The fulminant disease, intense viremia, and histopathologic changes observed in hamsters with PTV and GFV infections mimic those of severe RVF.1,9,11,18 Most investigators agree that the primary lesion in RVF is in the liver, although other organs are also affected. Hepatocyte necrosis, nuclear fragmentation, and eosinophilic inclusions are observed in the liver of animals and humans dying of RVF.1,9,18,19 Liver function test results are also abnormal in the disease, indicating hepatic dysfunction.1 Several investigators1,9,18,19 have noted that the histopathology of the liver in RVF is very similar to that seen in yellow fever, except that steatosis is minimal or FIGURE 5. Immunohistochemical staining for Punta Toro virus (PTV) and Gable Forest virus (GFV) antigens. A, Liver from a hamster infected with PTV showing a few perivascular hepatocytes with positive staining, and two hepatocytes with condensed dark nuclei with positive staining (arrowheads). B, Liver from a hamster infected with PTV showing more advanced focal necrosis. Most of the hepatocytes surrounding the focus of necrosis (arrow) are positive for viral antigens. C, Kidney from a hamster infected with GFV showing positivity in the epithelial cells of the renal tubules. D, Lung from a hamster infected with GFV showing only focal mild positivity in the interstitial cells in the septa. (Immunoperoxidase stained, magnification × 100.) 275 ANIMAL MODEL FOR PHLEBOVIRUS INFECTION absent. The results of our study, as well as those of previous one by Anderson and others,13 demonstrate the similarity of the pathology produced in hamsters by PTV, GFV, and RVF viruses. In the case of PTV13 and GFV (Figure 3), there appeared to be a direct correlation between the intensity of viremia and the degree of histopathology in the liver. Splenic necrosis and karyorrhexis were observed in the PTV-and GFV-infected hamsters. While most investigators have focused on the liver pathology associated with RVF, splenic hemorrhage, necrosis, and lymphoid depletion also occur in the disease.1,9,11 Within 24 hours after infection with PTV or GFV, approximately half of our hamsters showed IP. At 48−72 hours, all of the animals had IP, with histopathologic scores of 2−3. Pulmonary congestion and infiltration of the lungs with red blood cells and polymorphonuclear leukocytes have also been reported in animals with RVF.9 Periarteritis and mild IP were described previously by Anderson and others13 in their Punta Toro animal model. In human cases of RVF, clinical and pathologic evidence of IP had been occasionally reported from Rhodesia20 and Egypt,21 respectively. Histopathologic changes were observed in the adrenal glands of the PTV-infected hamsters. These were similar to those previously described,13 although this earlier report only noted involvement of the zona reticularis. The marked involvement of the adrenal cortex may have caused adrenal cortical insufficiency in the infected hamsters, since the zona glomerulosa is the main source of mineralocorticoids (aldosterone) and the zone reticularis is responsible for some of the glucocorticoids (cortisol). The resulting acute adrenal insufficiency probably contributes to death in the infected animals. Segmental mucosal necrosis involving the duodenum was observed in the PTV-infected hamsters. This type of “ischemic” necrosis was also described in PTV-infected hamsters by Anderson and others,13 who attributed the mechanisms to diffuse intravascular coagulation (DIC). In our opinion, this is not a credible explanation for the observed pathology, since DIC is a diffuse process and therefore should involve other segments of the gastrointestinal tract. In the case of the PTVinfected hamsters, the pathology was localized to the duodenum. This finding suggests that other viral effects may be involved in the observed small bowel necrosis. Nevertheless, these changes may serve as the pathologic basis for the gastrointestinal hemorrhages. It is well known that gastrointestinal bleeding occurs in laboratory and domestic animals infected with RVF virus.1,9,11 Profuse gastrointestinal hemorrhage occasionally occurs in human cases of RVF as well.1 Despite obvious histologic changes in the spleen, viral antigens could not be detected in this tissue immunohistochemically. In contrast, viral antigens were consistently identified in the liver, first in the perivascular zones, and later spread to discrete hepatocytes in other areas (mostly cells showing degenerative changes or apoptosis), and to more evident necrotic foci (Figure 5). Staining for viral antigens in the kidney was variable, showing positivity in epithelial cells in some of the tubules. Interestingly, although no positive staining was found in the adrenal glands in GFV-infected hamsters, zonal distribution of viral antigens was demonstrated in the adrenal glands in PTV-infected hamsters, corresponding to necrosis in these areas. Thus, the infection patterns of GFV and PTV in hamsters have similarities and differences in terms of organ involvement and antigen distribution. The results from the immunohistochemical analysis confirmed the establishment of viral infection in these models, and correlated the viral replication in the tissues to pathologic injuries. These results are encouraging because they demonstrate the feasibility of using this technique for tissue-based diagnosis of viral disease in archival tissue samples. In summary, the similarities of the clinical disease and pathology produced by these phleboviruses in hamsters suggest that PTV and GFV would be good alternative models for studying the pathogenesis of RVF and for testing potential therapeutic agents for the prevention and treatment of severe phlebovirus infection. Received February 27, 2003. Accepted for publication May 27, 2003. Financial support: This study was supported by the National Institutes of Health (grants AI-10984 and AI-50175 and contract NO1AI-25489). Ann F. Fisher was supported by a National Institutes of Health T32 training grant in Emerging and Re-emerging Infectious Disease (AI-07536). Authors’ addresses: Ann F. Fisher, Department of Internal Medicine, University of Texas Medical Branch, Galveston, TX 77555-0588. Robert B. Tesh, Jessica Tonry, Hilda Guzman, and Dongying Liu, Department of Pathology and Center for Biodefense and Emerging Infectious Diseases, University of Texas Medical Branch, Galveston, TX 77555-0588. Shu-Yuan Xiao, Department of Pathology and Center for Biodefense and Emerging Infectious Diseases, and Department of Internal Medicine, University of Texas Medical Branch, 301 University Boulevard, Galveston, TX 77555-0588, Telephone: 409772-8447, Fax: 409-772-4676, E-mail: [email protected]. REFERENCES 1. Gear JHS, 1988. Rift Valley fever. Gear JHS, ed. CRC Handbook of Viral and Rickettsial Hemorrhagic Fevers. Boca Raton, FL: CRC Press, 101−118. 2. Meegan JM, Bailey CL, 1989. Rift Valley fever. Monath TP, ed. The Arboviruses: Epidemiology and Ecology. Volume 5. Boca Raton, FL: CRC Press, 51−76. 3. Peters CJ, 1997. Emergence of Rift Valley fever. Saluzzo JF, Dodet B, eds. Factors in the Emergence of Arbovirus Disease. Paris: Elsevier, 253−264. 4. Elliott RM, Bouloy M, Calisher CH, Goldbach R, Moyer JT, Nichol ST, Pettersson R, Pluysnin A, Schmaljohn CS, 2000. Family Bunyaviridae. van Regenmortel MHV, Fauquet CM, Bishop DHL, Carstens EB, Estes MK, Lemon SM, Maniloff J, Mayo MA, McGeoch DJ, Pringle CR, Wickner RB, eds. Virus Taxonomy. Classification and Nomenclature of Viruses. Seventh Report of the International Committee on Taxonomy of Viruses. San Diego: Academic Press, 559−621. 5. Centers for Disease Control and Prevention, 2000. Outbreak of Rift Valley fever − Saudi Arabia, August-October, 2000. MMWR Morb Mortal Wkly Rep 49: 905–908. 6. Peters CJ, 2000. Are hemorrhagic fever viruses practical agents for biological terrorism? Sheld WM, Craig WA, Hughes JM, eds. Emerging Infections 4. Washington, DC: American Society for Microbiology Press, 201−209. 7. National Institute of Allergy and Infectious Diseases, 2002. The Counter-Bioterrorism Research Agenda of the National Institute of Allergy and Infectious Diseases (NIAID) for CDC Category A Agents. Bethesda: National Institutes of Health. 8. Centers for Disease Control and Prevention/ National Institutes of Health, 1999. Biosafety in Microbiological and Biomedical Laboratories . Fourth edition. Washington, DC: U.S. Government Printing Office. 9. Easterday BC, 1965. Rift Valley fever. Adv Vet Sci 10: 65–127. 10. Daubney R, Hudson J, Garnham P, 1931. Enzootic hepatitis or Rift Valley fever. An undescribed virus disease of sheep, cattle, and man from East Africa. J Pathol Bacteriol 34: 545– 579. 276 FISHER AND OTHERS 11. Findlay GM, 1932. Rift Valley fever or enzootic hepatitis. Trans R Soc Trop Med Hyg 25: 229–265. 12. Tesh RB, Duboise SM, 1987. Viremia and immune response with sequential phlebovirus infections. Am J Trop Med Hyg 36: 662–668. 13. Anderson G, Slayter M, Hall W, Peters CJ, 1990. Pathogenesis of a phleboviral infection (Punta Toro virus) in golden Syrian hamsters. Arch Virol 114: 203–212. 14. Karabatsos N, 1985. International Catalogue of Arboviruses Including Certain Other Viruses of Vertebrates. Third edition. San Antonio, TX: American Society of Tropical Medicine and Hygiene. 15. Xiao S-Y, Zhang H, Guzman H., Tesh R, 2001. Experimental yellow fever virus infection in golden hamster (Mesocricetus auratus): II. Pathology. J Infect Dis 183: 1437−1444. 16. Tesh R, Guzman H, Travassos da Rosa A, Vasconcelos P, Dias L, Bunnell J, Zhang H, Xiao S-Y, 2001. Experimental yellow fever virus infection in the golden hamster (Mesocricetus aura- 17. 18. 19. 20. 21. tus). I. Virologic, biochemical and immunologic studies. J Infect Dis 183: 1431–1436. Xiao S-Y, Guzman H, Travassos da Rosa APA, Zhu H-B, Tesh RB, 2003. Alteration of clinical outcome and histopathology of yellow fever virus infection in a hamster model by previous infection with heterologous flaviviruses. Am J Trop Med Hyg 68: 695−703. Mims CAC, 1957. Rift Valley fever in mice. VI. Histological changes in the liver in relation to virus multiplication. Aust J Exp Biol Med Sci 35: 595–604. Findlay GM, 1933. Cytological changes in the liver of Rift Valley fever, with special reference to the nuclear inclusions. Br J Exp Pathol 14: 207–219. Swaneppoel R, Manning B, Watt JA, 1979. Fatal Rift Valley fever of man in Rhodesia. Centr Afri J Med 25: 1−8. Abdel-Wahab KSED, Baz LME, 1978. Rift Valley fever virus infections in Egypt: pathological and virological findings in man. Trans R Soc Trop Med Hyg 72: 392–396.