* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Prof_Elias_Inorg_lec_6
Aromaticity wikipedia , lookup
Discodermolide wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Asymmetric induction wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Aromatization wikipedia , lookup
Cluster chemistry wikipedia , lookup
Hydroformylation wikipedia , lookup
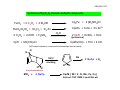
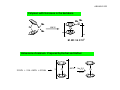
AJELIAS L2‐S5 Infrared Spectroscopy‐ A spectro‐analytical tool in chemistry Infrared (IR) spectroscopy is one of the most common spectroscopic techniques used by organic and inorganic chemists. Simply, it is the absorption measurement of different IR frequencies by a compound positioned in the path of an IR beam. The main goal of IR spectroscopic analysis is to determine the chemical functional groups in the sample. Functional groups are identified based on vibrational modes of the groups such a stretching, bending etc. Different vibrational modes absorb characteristic frequencies of IR radiation. An infrared spectrophotometer is an instrument that passes infrared light through a molecule and produces a spectrum that contains a plot of the amount of light transmitted on the vertical axis against the wavelength of infrared radiation on the horizontal axis. Absorption of radiation lowers the percentage transmittance value. AJELIAS L2‐S6 Infrared Spectroscopy‐ Spectra of Metal Carbonyls OC OC OC CO OC CO Mn CO Mn The range in which the band appears decides bridging or terminal . CO CO OC terminal The number of bands is only related to the symmetry of the molecule OC OC OC O C O C Fe CO Fe C O CO CO terminal bridging AJELIAS L2‐S7 Terminal versus bridging carbonyls O M O C C C M M M M O M terminal ν CO 2120-1850 cm-1 bridging μ 2 1850-1700 cm-1 bridging μ 3 1730-1620 cm-1 Cp Fe CO CO OC OC Cr OC Fe Cp OC CO Fe CO CO 2000 cm‐1 Fe Cp 2018, 1826 cm‐1 CO 1620 cm‐1 Cp AJELIAS L2‐S8 Factors which affect νCO stretching frequencies 1.Charge on the metal 2. Effect of other ligands Variation in νCO (cm–1) of the first row transition metal carbonyls free CO 2143 As the electron density on a metal centre increases, more π‐back‐bonding to the CO ligand(s) takes place. This weakens the C–O bond further as more electron density is pumped into the empty π* anti‐bonding carbonyl orbital. This increases the M–C bond order and reduces the C‐O bond order. That is, the resonance structure M=C=O becomes more dominant. Ni(CO)4 2057 Co(CO)41890 Co2(CO)8 2044(av, ter) [Fe(CO)4]21815 Fe(CO)5 2030 [Mn(CO)4]31600,1790 Mn(CO)6 + 2098 [Cr(CO)4]41462,1657 Cr(CO)6 2000 V(CO)6¯ 1860 Mn2(CO)10 2013 (av) V(CO)6 1976 Ti(CO)621747 M C ν O CO Higher M C ν O CO Lower More back bonding AJELIAS L2‐S9 Other spectator ligands: Phosphines χ(cm–1) PR3 νCO, (cm–1) 2056.1 0.0 PPh2(C6F5) 2074.8 18.7 PCy3 2056.4 0.3 P(OEt)3 2076.3 20.2 P(i-Pr)3 2059.2 3.1 P(p-C6H4-CF3)3 2076.6 20.5 PEt3 2061.7 5.6 P(OMe)3 2079.5 23.4 P(NMe2)3 2061.9 5.8 PH3 2083.2 27.1 PMe3 2064.1 8.0 P(OPh)3 2085.3 29.2 PBz3 2066.4 10.3 P(C6F5)3 2090.9 34.8 P(o-Tol)3 2066.6 10.5 PCl3 2097.0 40.9 PPh3 2068.9 12.8 PF3 2110.8 54.7 PPh2H 2073.3 17.2 P(CF3)3 2115.0 58.9 PR3 νCO, (cm–1) P(t-Bu)3 Δ νCO wrt P(t-Bu)3 χ(cm–1) Δ νCO wrt P(t-Bu)3 PR3 Lowest CO stretching frequency Most donating phosphine best σ donor OC Ni Highest CO stretching frequency Least donating phosphine CO best π acceptor CO AJELIAS L2‐S10 Effect of a ligands trans to CO Effect of different co-ligands on νCO (cm-1) of Mo(CO)3L3 CO L CO Mo L CO L Complex (fac isomers) ν CO cm–1 Mo(CO)3(PF3)3 2090, 2055 Mo(CO)3(PCl3)3 2040, 1991 Mo(CO)3[P(OMe)3]3 1977, 1888 Mo(CO)3(PPh3)3 1934, 1835 Mo(CO)3(NCCH3)3 1915, 1783 Mo(CO)3(dien)* 1898, 1758 Mo(CO)3(Py)3 1888, 1746 More back bonding = More lowering of the C=O bond order = More lower ν CO stretching frequency With each negative charge added to the metal centre, the CO stretching frequency decreases by approximately 100 cm–1. The better the σ donating capability of the other ligands on the metal, more electron density given to the metal, more back bonding (electrons in the antibonding orbital of CO) and lower the CO stretching frequency. AJELIAS L2‐S11 Synthesis of Metal Carbonyls Direct carbonylation Reductive carbonylation AJELIAS L2‐S12 Reactions of Metal Carbonyls Reduction : Carbonyl anions V(CO)6 + Na Mn2(CO)10 Co2(CO)8 Na[V(CO)6] 2 Na[Mn(CO)5] + 2Na 2 Na[Co(CO)4] + 2Na Fe(CO)5 + Na/Hg Na 2Fe(CO)4 Oxidation : Iodocarbonyls Mn2(CO)10 Fe(CO)5 + I2 2 Mn(CO)5I + I2 Fe(CO)4I2 Photochemical substitution W(CO)6 + PPh3 Fe(CO)5 + hν W(CO)5(PPh3) + CO hν Fe(CO)3 + 2CO In the presence of UV radiation a monodentate ligand displaces only one CO unit AJELIAS L2‐S13 Reactions of Metal Carbonyls Nucleophilic addition to CO R CO OC OCH 3 C CO W OC R OLi OC RLi CO ether C CO W OC OC OC [Me 3O]BF 4 CO W OC CO CO OC OC Fischer Carbene Carbenes are catalysts for olefin metathesis Migratory insertion of CO Me Me OC C CO Mn OC CO OC O CO high pressure OC CO Mn OC CO OC Problem solving ‐ synthesis Give a scheme for the synthesis of Mn(CO)4(PPh3)[C(O)CH3] starting from Manganese acetate, Mn(OAc)2. 2 Mn(OAc)2 + 4 Na + 10 CO high temp high pressure Mn2(CO)10 + 4 NaOAc Mn2(CO)10 + 2 Na 2 NaMn(CO)5 NaMn(CO)5 + CH3I CH3Mn(CO)5 CH3Mn(CO)5 + CO CH3C(O)Mn(CO)5 + PPh3 CH3C(O)Mn(CO)5 ( migratory insertion) CH3C(O)Mn(CO)4PPh3 hv Or at step 3 direct reaction with acyl chloride instead of MeI. Step 1 other reducing agents e.g. AlEt3 can also be used. AJELIAS L2‐S14 Metal‐ Sandwich compounds Hapticity of sandwich compounds varies from 1‐8 AJELIAS L2‐S15 Why metal – sandwich compounds are important? 1. Transition metal/ metal ion embedded inside an organic matrix: Makes a metal ion soluble even in hydrocarbon solvents. E.g. Ferrocene is soluble in hexane while Fe2+ as such is not. Outcome: a hydrocarbon soluble additive/catalyst 2. Coordination to an electropositive metal often changes the reactivity and electronic properties of the π system bound to it (benzene vs ferrocene) 3. A stericially protected metal site where a wide range of catalytic applications are possible on the. e.g alkene polymerization 4. Metal sandwich compounds are excellent substrates to make planar chiral compounds. Applications as chiral catalysts in asymmetric catalysis X X Y Y Fe Planr chirality: Non‐ super‐imposable mirror images Fe AJELIAS L2‐S16 Cyclopentadienyl (Cp−) • Cyclopentadienyl (Cp−) the most important of all the polyenyl ligands • It gets firmly bound to the metal • generally inert to nucleophilic reagents. • used as a stabilising ligand for many complexes. M η1 M η3 Least com m on M η5 m ost com m on (η5‐Cp)(η3‐Cp)W(CO)2 •Neutral cyclopentadiene (C5H6) is a weak acid with a pKa of around 15 •Deprotonated with strong base or alkali metals to generate the anionic Cp− AJELIAS L2‐S17 Synthesis of Cp (C5H5‐) based sandwich compounds Cp2Fe + 2 [Et2NH2]Cl FeCl2 + 2 C5H6 + 2 Et2NH Cp2Ru + C5H8 + 3/2 Zn2+ RuCl3(H2O)n + 3C5H6 + 3/2 Zn H 2O 2 C5H6 + 2 KOH + Tl2SO4 2 CpTl + K2SO4 + H2O (poisonous) CpTl + Mn(CO)5Cl CpMn(CO)3 + TlCl + 2 CO CpTl based chemistry is not practiced nowadays due to toxicity H H Na 180°C 2 NaCp + H2 cracking H H H H dicyclopentadiene MCl2 + 2 NaCp Cp2M [ M = V, Cr, Mn, Fe, Co] Solvent: THF, DME, Liquid NH3 etc AJELIAS L2‐S18 Ferrocene: synthesis Fe Lab Synthesis Fe + 2 (R 3NH)Cl FeCl2 + 2 R 3N + H 2 FeCl2 + 2 C 5H 6 + 2 R 3 N Cp 2Fe + 2(R 3NH)Cl FeCl2 + 2 NaCp Cp2Fe AJELIAS L2‐S19 Reactions of Ferrocene Ferrocene undergoes electrophilic substitution reactions. Many of its reactions are faster than similar reactions of benzene Necessary requirement: The electrophile should not be oxidizing in nature I2 Fe FeCp2 + HBF4.OEt2 Fe p- benzoquinone Et2O FeCl3 FeCp2 + NH4PF6 H2O/Acetone I3 [FeCp2][BF4] [FeCp2][PF6] The oxidized Cp2Fe+, ferrocenium cation, will repel the electrophile away. Therefore direct nitration, halogenation and similar reactions cannot be carried out on ferrocene. Acetylation H3C(O)C Ac2O/ H3PO4 Fe 60 min, 50 °C Fe CH3C(O)Cl AlCl3(1:2:2) C(O)CH3 C(O)CH3 C(O)CH3 Fe Fe C(O)CH3 90 % 90 % traces 3.3 x 106 times faster than benzene AJELIAS L2‐S20 Chloromercuration (hazardous) Hg(OAc) Hg(OAc)2 Fe HgCl LiCl Fe Fe Br2/I2 Br, I derivatives 109 times faster than benzene Mannich reaction H2 C NR2 HCHO/R2NH Fe Fe H3PO4 Does not happen with benzene; only with phenols/anilines Lithiation reaction Li Fe Li t-BuLi Fe n-BuLi N Fe TMEDA Li N (3:2 adduct) Does not happen with benzene; only with bromobenzene AJELIAS L2‐S21 Lithiation and 1,1’‐di‐lithiation – access to range of new derivatives Li CO2/H+ HOOC SiCl4 Fe Cl3Si Fe Fe ( ) 3B O Bu 1/8 S 8 H+ (HO)2B SLi I2 Fe Fe I Fe NaCN CN Fe dppf [1]ferrocenophane AJELIAS L2‐S22 Polymers with ferrocene in the backbone Me Me Si Me Fe 130 °C Si Fe Me n M. Wt: 3.4 X 105 Bisbenzene chromium: Prepared by Fischer and Hafner 3 CrCl3 + 2 Al + AlCl3 + 6 C6H6 3 Cr AlCl4 Na2S2O4 KOH Cr Problem solving ‐ synthesis Starting fro m ferrocene show minimum number of steps for preparing 1,1’‐ ferrocene dicarboxylic acid





























![[1,1`- Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](http://s1.studyres.com/store/data/006759823_1-98a349852ea902f818cbb367d542f255-150x150.png)