* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Editorial Heart Failure A PKGarious Balancing Act - VU-dare
Remote ischemic conditioning wikipedia , lookup
Coronary artery disease wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Electrocardiography wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Heart failure wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
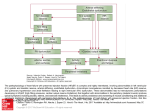
Heart Failure : A PKGarious Balancing Act David A. Kass Circulation. 2012;126:797-799; originally published online July 17, 2012; doi: 10.1161/CIRCULATIONAHA.112.124909 Circulation is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231 Copyright © 2012 American Heart Association, Inc. All rights reserved. Print ISSN: 0009-7322. Online ISSN: 1524-4539 The online version of this article, along with updated information and services, is located on the World Wide Web at: http://circ.ahajournals.org/content/126/7/797 Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in Circulation can be obtained via RightsLink, a service of the Copyright Clearance Center, not the Editorial Office. Once the online version of the published article for which permission is being requested is located, click Request Permissions in the middle column of the Web page under Services. Further information about this process is available in the Permissions and Rights Question and Answer document. Reprints: Information about reprints can be found online at: http://www.lww.com/reprints Subscriptions: Information about subscribing to Circulation is online at: http://circ.ahajournals.org//subscriptions/ Downloaded from http://circ.ahajournals.org/ at Vrije Universiteit--Amsterdam on March 12, 2013 Editorial Heart Failure A PKGarious Balancing Act David A. Kass, MD T he pathologically hypertrophied and failing heart is a battlefield in a war that would make even George Lucas proud. On the one side, you have hemodynamic, neurohormonal, morphological, and cellular/molecular dark forces urging the ventricle toward decompensation and ultimate demise. On the other, Jedi signaling cascades try valiantly to stave off the impending disaster. Alas, unlike the movies, the dark side often wins, and we need better-equipped counterforces to change this. Current heart failure therapies are fairly defensive— blocking neurohumoral stimuli and hemodynamic overload. However, adaptive/offensive strategies are advancing, including those aimed at enhancing metabolism, vascular supply, and cell regeneration, and those activating molecular signaling to counter maladaptation. Article see p 830 An example of disease-countering (Jedi) signaling is that related to cyclic GMP-dependent protein kinase (PKG). cGMP and its cousin cAMP are second-messenger molecules involved in a broad array of cell signaling. In the heart, the role of cAMP is firmly established, with localized signaling targeting protein kinase A to modulate excitation– contraction coupling and metabolism, and exchange proteins activated by cAMP (Epacs) to mediate chronic stress. Although sustained cAMP activation contributes to maladaptive stress, cGMP activation of PKG blunts these responses. cGMP is synthesized in heart muscle by 2 pathways—nitric oxide-stimulated soluble guanylate cyclase (sGC) and a natriuretic peptide receptor-coupled cyclase. Once generated, cGMP binds to regulatory domains in PKG to activate the kinase and to affect signaling. There is no known Epac equivalent for cGMP. cGMP is catabolized by members of the phosphodiesterase (PDE) superfamily, and by its binding to regulatory (PDE2 and PDE5) or catalytic (PDE3) domains in several of these proteins; cGMP also regulates its own hydrolysis and that of cAMP.1 Unlike in the vasculature, where resting cGMP and corresponding PKG activity contribute to vascular tone and endothelial function, basal activity of this signaling cascade in cardiac myocytes is very low. PKG knock-down or loss-ofThe opinions expressed in this article are not necessarily those of the editors or of the American Heart Association. From The Johns Hopkins University School of Medicine, Baltimore, MD. Correspondence to David A. Kass, MD, Division of Cardiology, Johns Hopkins Medical Institutions, Ross Building 858, 720 Rutland Avenue, Baltimore, MD 21205. E-mail [email protected] (Circulation. 2012;126:797-799.) © 2012 American Heart Association, Inc. Circulation is available at http://circ.ahajournals.org DOI: 10.1161/CIRCULATIONAHA.112.124909 function studies have found minimal impact on either cardiac morphology or function under resting conditions,2,3 and some even questioned its role in the heart.4 However, similar to an automotive brake, the influence of cGMP and PKG in hearts under stress is greater. During the past decade, studies have shown that cGMP/PKG activation counters a broad array of acute and chronic cardiac stress responses, including those from -adrenergic stimulation,5 ischemic injury,6 pressure and volume overload,7,8 and doxorubicin cardiotoxicity.9 Methods to activate cGMP/PKG signaling have already been developed as clinical therapies, including those that enhance cGMP synthesis, such as organic nitrates (eg, isosorbide dinitrate), activate guanylate cyclase (eg, cinaciguat or natriuretic peptides), and curtail cGMP hydrolysis (eg, sildenafil, tadalafil). All are either used or being studied in patients with heart failure. PDE5 inhibitors were first thought to have negligible effects on the heart; however, research during the past decade has revealed substantial benefits in a variety of experimental and human cardiac diseases. Results of single-center trials testing sildenafil in patients with dilated heart failure indicated improvements in symptoms and exercise capacity, microvascular function, pulmonary hypertension, and cardiac morphology and function.10,11 The PhosphodiesteRasE-5 Inhibition to Improve Quality of Life And EXercise Capacity in Diastolic Heart Failure (RELAX) trial of 225 patients, a National Institutes of Health–sponsored multicenter study testing the utility of sildenafil in patients with heart failure and a preserved ejection fraction (HFpEF), completed enrollment in early 2012, and results are anticipated later this year. Another multicenter National Institutes of Health trial of 2000 patients, PDE5 Inhibition with Tadalafil Changes Outcomes in Heart Failure (PITCH-HF), will start shortly and will test the efficacy of tadalafil in patients with heart failure and a reduced ejection fraction (HFrEF) who also have resting or exercise-induced pulmonary hypertension. On the stimulation side, the combination of isosorbide dinitrate and hydralazine is approved for treating HFrEF in blacks, direct sGC activators are currently being tested in HFrEF, and B-type and more recently, CD-type natriuretic peptides are being examined as chronic therapies. The efficacy of treatments engaging the cGMP/PKG pathway depends on specifics of the signaling, and this itself can be modified by heart disease. For example, a nitric oxide (NO) mimetic leverages sGC to generate cGMP. However, in vascular and cardiac disease, sGC is often subject to oxidative stress that reduces its responsiveness to NO.12,13 Direct sGC agonists that are heme (oxidative state) independent may circumvent this limitation.13 Generating more cGMP becomes less effective if cGMP-PDEs are upregulated, and in this setting, inhibiting the appropriate PDEs becomes very useful. Neither elevated cGMP synthesis nor hydrolysis is a Downloaded from http://circ.ahajournals.org/ at797 Vrije Universiteit--Amsterdam on March 12, 2013 798 Circulation August 14, 2012 feature of the normal heart, but it is in human HFrEF14,15 and experimental pressure overload, supporting current efforts with PDE5 inhibition. Last, PKG itself needs to have something useful to do; that is, a targetable pathway must exist in which modification by the kinase can offset cardiac maladaptation. Some diseases activate these pathways more than others. To date, PKG modulation of the calcineurin/nuclear factor of activated T-cells signaling cascade, transient receptor potential channel 6, mitochondrial ATP-sensitive potassium channel, Ras homolog gene family, mamber A, regulator of G-protein signaling 2 and 4, and other factors have all been identified as key contributors to its amelioration of cardiac disease.16 Thus, whether intrinsic cGMP/PKG signaling works as a competent Jedi knight that can be enhanced by therapeutic interventions depends on a balancing act among factors controlling the signaling cascade. In the current issue of Circulation, van Heerebeek et al17 present provocative new data regarding this balance, and in particular show how things go awry in HFpEF. The data set is unique—left ventricular endocardial biopsies from nearly 150 patients with nonischemic HFrEF, HFpEF, or aortic stenosis (the latter obtained at surgery from the left ventricular outflow tract). The measurements include passive tension in isolated cardiac myocytes and assays to assess various levels, activity, and posttranslational modifications of proteins involved with the NO/natriuretic peptide– cGMP/PKG cascade. As these investigators reported previously,18 maximal passive myocyte stiffness is higher in HFpEF than HFrEF (in the new study, they find it higher than in aortic stenosis [AS] patients as well). Their earlier study showed this disparity could be eliminated by incubation with PKA, and in the current research they find a similar result using PKG, which suggests a deficit of PKG activity in HFpEF that is supported more directly by several kinase assays. With regard to why PKG activity might be less in HFpEF, the investigators find cGMP is also much reduced. This correlates with lower pro-brain natriuretic peptide expression; however, levels are similarly depressed in AS patients whose PKG activity is much higher. PDE5 protein expression appears similar among groups. Rather, van Heerebeek et al18 highlight greater oxidative/nitrosative stress in HFpEF, suggesting reduced NO-stimulated cGMP synthesis as the culprit. The authors also perform subgroup analysis, examining myocyte passive stiffness, PKG activity, and cGMP levels in each group with or without diabetes mellitus (DM). Patients with HFpEF have the highest stiffness regardless of DM status. However, unlike DM– patients, both HFpEF and AS with DM⫹ similarly reduce PKG activity, raising questions about its role. Furthermore, despite low PKG activity in patients with AS and DM, these subjects have greater cGMP levels unlike patients with HFpEF and DM. These investigators have shown previously that DM exacerbates myocyte passive stiffness in patients with AS,19 and this seems true in the current analysis, although it is not mentioned. PKG activity also appears to be about half in DM-AS versus AS alone, although significance is not noted. The current study by van Heerebeek et al17 poses an intriguing new explanation for HFpEF pathophysiology. Reduced PKG activity in stressed myocardium would be antic- ipated to exacerbate the pathophysiology of HFpEF, not only through effects on passive properties but also on remodeling and on systole. In mice harboring myocyte-targeted and controllable PDE5 expression, upregulation (lowered PKG activity) worsened hypertrophy, function, and fibrosis in a pressure overload model; reducing PDE5 expression (increasing PKG activity) did the opposite.2 Although a prior genetic loss-of-function model concluded PKG was unimportant to chronic cardiac stress remodeling,4 more recent studies,2 including one from some of the same investigators3 using a more direct genetic approach to suppress PKG in myocytes, support an important protective role. The finding of similar improvement in myocyte stiffness in HFpEF (and HFrEF) to PKA (prior data18) and now to PKG suggests the residue targets may be similar. Titin, for example, serves as a molecular spring, and both PKG and PKA modify the same residues in the variable N2b region that reduce its stiffness constant.20 The previous work18 raised questions about the use of -blockade in HFpEF, given its suppression of PKA. Rather than stimulate PKA further, however, the current study suggests a similar benefit can be obtained by cGMP/PKG stimulation. van Heerebeek et al17 speculate that oxidation of titin may also play a role in HFpEF, although the rescue of stiffening by PKG alone in vitro argues for phosphorylation as a dominant mechanism. The data also raise intriguing questions regarding how one might increase PKG activity to treat HFpEF. Although PDE5 immunohistochemical staining is similar among the groups in the current study, others have reported substantial upregulation of PDE5 expression in HFrEF compared with low levels in normal control subjects.14,15 Thus, the current data could be consistent with upregulation in all groups. PDE5 activity was not assessed, and expression does not always reflect enzyme activity as a result of posttranslational changes and alterations in protein localization and function (eg, hydrolysis of cGMP generated by sGC versus natriuretic peptide receptor-coupled cyclase).21 Last, the left ventricular biopsy analysis reflects limited sampling, whereas prior studies supporting PDE5 upregulation have examined tissue from explanted hearts.14,15 Relatively low pro-brain natriuretic peptide is observed in HFpEF myocardium, and although this may or may not have contributed to the decline in cGMP, natriuretic peptide stimulation still seems a reasonable therapeutic option. The mechanism favored by van Heerebeek et al17 is that NOreactive oxygen species (ROS) interactions rose in HFpEF, as reflected by enhanced tissue nitrotyrosine, presumably reflecting diminished nitric oxide synthase-NO-cGMP synthesis. This is interesting, although still speculative. Specifically, confirmation that nitric oxide synthase-derived NO and cGMP were indeed suppressed more prominently (and ROS enhanced reciprocally) in HFpEF was not demonstrated. The nitrotyrosine assay has limitations, one being a lack of specificity for peroxinitrite formation. Although the authors suggest that a greater prevalence of DM may have contributed to redox imbalance in HFpEF, their subset analysis found a similar depression of cGMP and PKG activity in HFpEF with or without DM. It would have been interesting to see nitrotyrosine data for these 2 subgroups. Other studies have found ROS activation and ROS/NO imbalance in Downloaded from http://circ.ahajournals.org/ at Vrije Universiteit--Amsterdam on March 12, 2013 Kass HFrEF models; indeed, this has been suggested as pertinent to patients who respond to isosorbide dinitrate and hydralazine.22 If HFpEF reflects greater NO-ROS imbalance, then perhaps direct heme-independent sGC activators such as cinaciguat would be a better choice, given likely redox changes in sGC that blunt its NO response. As more and more therapeutic avenues become available to modulate cGMP/PKG signaling, interest in this pathway and ways to leverage it for treating heart disease will continue to increase. The recent promising results from PDE5 inhibitor trials in HFrEF, and evidence of potency in HFpEF as well, are moving this field forward, with pivotal clinical trials now underway. To date, natriuretic peptide therapy has been used subacutely, given the need for intravenous administration. Whether brain natriuretic peptide or newer, more stable and potent natriuretic peptides are beneficial when used chronically remains to be tested. Last, novel methods to improve nitric oxide synthase activity or sGC generation of cGMP are moving forward. Understanding how to use these approaches best requires appreciation of the balance between cGMP synthesis, hydrolysis, and PKG targeting. The Paulus Laboratory and colleagues are to be congratulated for providing us with valuable new insights in this regard. Acknowledgments Dr Kass thanks Eiki Takimoto for his helpful review. This work was supported by National Institutes of Health grants HL077180, HL-089297, and HL-093432; Muscular Dystrophy Association Award 186454; and Fondation Leducq. Disclosures None. References 1. Francis SH, Blount MA, Corbin JD. Mammalian cyclic nucleotide phosphodiesterases: molecular mechanisms and physiological functions. Physiol Rev. 2011;91:651– 690. 2. Zhang M, Takimoto E, Hsu S, Lee DI, Nagayama T, Danner T, Koitabashi N, Barth AS, Bedja D, Gabrielson KL, Wang Y, Kass DA. Myocardial remodeling is controlled by myocyte-targeted gene regulation of phosphodiesterase type 5. J Am Coll Cardiol. 2010;56:2021–2030. 3. Frantz S, Klaiber M, Baba HA, Oberwinkler H, Volker K, Gassner B, Bayer B, Abesser M, Schuh K, Feil R, Hofmann F, Kuhn M. Stressdependent dilated cardiomyopathy in mice with cardiomyocyte-restricted inactivation of cyclic GMP-dependent protein kinase I. Eur Heart J. December 23, 2011. DOI:10.1093/eurheartj/ehr445. http://eurheartj. oxfordjournals.org. Accessed December 23, 2011. 4. Lukowski R, Rybalkin SD, Loga F, Leiss V, Beavo JA, Hofmann F. Cardiac hypertrophy is not amplified by deletion of cGMP-dependent protein kinase I in cardiomyocytes. Proc Natl Acad Sci U S A. 2010;107: 5646 –5651. 5. Borlaug BA, Melenovsky V, Marhin T, Fitzgerald P, Kass DA. Sildenafil inhibits beta-adrenergic-stimulated cardiac contractility in humans. Circulation. 2005;112:2642–2649. 6. Kukreja RC, Salloum FN, Das A. Cyclic guanosine monophosphate signaling and phosphodiesterase-5 inhibitors in cardioprotection. J Am Coll Cardiol. 2012;59:1921–1927. 7. Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, Bedja D, Gabrielson KL, Wang Y, Kass DA. Chronic inhibition of cyclic GMP phosphodiesterase 5a prevents and reverses cardiac hypertrophy. Nat Med. 2005;11:214 –222. A PKGarious Balancing Act 799 8. Kim KH, Kim YJ, Ohn JH, Yang J, Lee SE, Lee SW, Kim HK, Seo JW, Sohn DW. Long-term effects of sildenafil in a rat model of chronic mitral regurgitation: benefits of ventricular remodeling and exercise capacity. Circulation. 2012;125:1390 –1401. 9. Koka S, Das A, Zhu SG, Durrant D, Xi L, Kukreja RC. Long-acting phosphodiesterase-5 inhibitor tadalafil attenuates doxorubicin-induced cardiomyopathy without interfering with chemotherapeutic effect. J Pharmacol Exp Ther. 2010;334:1023–1030. 10. Guazzi M, Vicenzi M, Arena R, Guazzi MD. PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: results of a 1-year, prospective, randomized, placebo-controlled study. Circ Heart Fail. 2011;4:8 –17. 11. Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation. 2011;124: 164 –174. 12. Tsai EJ, Liu Y, Koitabashi N, Bedja D, Danner T, Jasmin JF, Lisanti MP, Friebe A, Takimoto E, Kass DA. Pressure-overload-induced subcellular relocalization/oxidation of soluble guanylyl cyclase in the heart modulates enzyme stimulation. Circ Res. 2012;110:295–303. 13. Stasch JP, Pacher P, Evgenov OV. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation. 2011;123:2263–2273. 14. Shan X, Quaile MP, Monk JK, French B, Cappola TP, Margulies KB. Differential expression of PDE5 in failing and nonfailing human myocardium. Circ Heart Fail. 2012;5:79 – 86. 15. Pokreisz P, Vandenwijngaert S, Bito V, Van den BA, Lenaerts I, Busch C, Marsboom G, Gheysens O, Vermeersch P, Biesmans L, Liu X, Gillijns H, Pellens M, Van LA, Buys E, Schoonjans L, Vanhaecke J, Verbeken E, Sipido K, Herijgers P, Bloch KD, Janssens SP. Ventricular phosphodiesterase-5 expression is increased in patients with advanced heart failure and contributes to adverse ventricular remodeling after myocardial infarction in mice. Circulation. 2009;119:408 – 416. 16. Zhang M, Kass DA. Phosphodiesterases and cardiac cGMP: evolving roles and controversies. Trends Pharmacol Sci. 2011;32:360 –365. 17. van Heerebeek L, Hamdani N, Falcão-Pires I, Leite-Moreira AF, Begieneman MPV, Bronzwaer JGF, van der Velden J, Stienen GJM, Laarman GJ, Somsen A, Verheugt FWA, Niessen HWM, Paulus WJ. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation. 2012;126:830 – 839. 18. van Heerebeek L, Borbely A, Niessen HW, Bronzwaer JG, van der Velden J, Stienen GJ, Linke WA, Laarman GJ, Paulus WJ. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation. 2006;113:1966 –1973. 19. Falcao-Pires I, Hamdani N, Borbely A, Gavina C, Schalkwijk CG, van der Velden J, van Heerebeek L, Stienen GJ, Niessen HW, Leite-Moreira AF, Paulus WJ. Diabetes mellitus worsens diastolic left ventricular dysfunction in aortic stenosis through altered myocardial structure and cardiomyocyte stiffness. Circulation. 2011;124:1151–1159. 20. Bishu K, Hamdani N, Mohammed SF, Kruger M, Ohtani T, Ogut O, Brozovich FV, Burnett JC Jr, Linke WA, Redfield MM. Sildenafil and B-type natriuretic peptide acutely phosphorylate titin and improve diastolic distensibility in vivo. Circulation. 2011;124:2882–2891. 21. Zhang M, Takimoto E, Lee DI, Santos CX, Nakamura T, Hsu S, Jiang A, Nagayama T, Bedja D, Yuan Y, Eaton P, Shah AM, Kass DA. Pathological cardiac hypertrophy alters intracellular targeting of PDE5 from nitric oxide synthase-3 to natriuretic peptide signaling. Circulation. July 24, 2012. DOI: 10.1161/CIRCULATIONAHA.112.090977. http:// www.circ.ahajournals.org. Accessed July 28, 2012. 22. Nediani C, Raimondi L, Borchi E, Cerbai E. Nitric oxide/reactive oxygen species generation and nitroso/redox imbalance in heart failure: from molecular mechanisms to therapeutic implications. Antioxid Redox Signal. 2011;14:289 –331. KEY WORDS: Editorials 䡲 cyclic nucleotides 䡲 heart failure 䡲 left ventricular diastolic dysfunction 䡲 oxidative stress 䡲 protein kinase G Downloaded from http://circ.ahajournals.org/ at Vrije Universiteit--Amsterdam on March 12, 2013